
Most salt affected soils occur in the arid and semi arid climates where rainfall is not sufficient to dissolve and leach salts such as Cl, SO4, HCO3, Na, K, Ca and Mg below the root zone. They are prevalent in irrigated region where improper irrigation and drainage methods are used. Sodium is of particular, since it can be toxic and detrimental to soil structure.
Salt problem in soil usually develop from:
1. Salt already present in the soil
2. High ground water table
3. Salt added to irrigation water
In low lying coastal areas, salt affected soil result from poor drainage practices, salt water inclusions, poor quality irrigation water, and submergence.
Water salinity is the amount of salt contained in the water. It is also called the “salt concentration” and may be expressed in grams of salt per litre of water (grams/litre or g/l) or in milligrams per litre (which is the same as parts per million, p.p.m).
The major effect of soil salinity on plant growth is that it affects water absorption by the plants. Even with sufficient soil moisture, crops still find it difficult to absorb enough water and die due to the inability to take up enough water.
The most common measurement of salt affected soils involve the amount of exchangeable sodium. The exchangeable sodium ration (ESR) is defined as
Exchangeable Na
————————————- (unit is meq/100g)
Exchangeable (Ca +Mg)
For measurement in solution, the sodium absorption ration (SAR) is commonly used. The concentration of sodium relative to calcium and magnesium in the soil is called the sodium adsorption ratio (SAR). SAR is a measure of soil sodicity.
Na
SAR=————————————- ( unit in meq/liter)
√( Ca + Mg )
————————
2
The cation concentrations units are mmol(+)/L. Soil water extracts with SAR values >13 indicate that the soil has sodium problem. At times, when SAR values >8, this result in relatively high concentrations of Na relative to Ca and Mg, results in dispersion of clay particles, soil structural breakdown, and soil pore blockage which reduces infiltration rates and increases erosion potential.
ESR is related emperically to SAR by
ESR=0.015 (SAR)
Exchangeable sodium percentage which is used for classifying salt affected soil is related to ESR by
ESP= 100(ESR)
——————
1 + ESR
The predominant anion in soil salinity include bicarbonate, carbonates, sulphates, and chloride. While the cations include sodium, calcium magnesium and little potassium.
CLASSIFICATION OF SOIL SALINIZATION
1. CLASSIFICATION OF SOIL BASED ON SALINITY AND ALKALINITY
______________________________________________
Degree of salinity. % by weight
——————————————————————————–
Free of salt. < 0.15%
Slightly salinity. 0.15 – 0.35%
Moderately salinity. 0.35 – 0.65%
Strongly saline. >0.65%
______________________________________________
__________________________________________________
Degree of alkalinity % of total exchangeable ions
————————————————————————–
Slightly alkaline. <20% exchangeable sodium
Moderately alkaline. 20 -50% exchangeable
. sodium
Strongly alkaline >50% exchangeable sodium
——————————————————————————-
2. CLASSIFICATION OF SALT AFFECTED SOIL
Salt affected soils are classified into three categories according to the total soluble salt (measured by electrical conductivity), pH and exchangeable sodium percentage as shown below. They are categorized as saline, sodic and saline-sodic, depending on the content.
Electrical Conductivity units is μS/cm = microseimens per centimetre
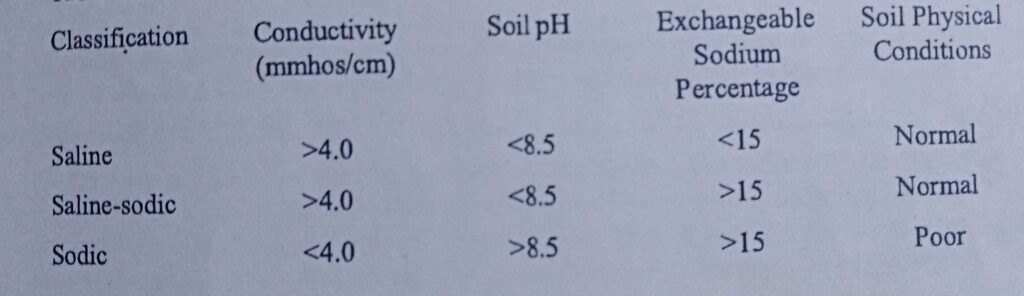
1 SALINE SOIL: Saline soils are soils with high enough soluble salts in the soil solution which negatively affect plant growth. They are also called white alkaline soil because of the depositing of salts found on soil surface following evaporation. The excess salt mostly found as Na, Ca and Mg sulfates or chloride, can be leached out with little increase in pH.
Usually, saline soils have good physical structure and water infiltration and soil permeability is not affected. They have physicochemical characteristics which include:
Electrical Conductivity: >4 ds m-1
Exchangeable Sodium Percentage: <15%
Sodium Adsorption Ratio: <13%
pH: <8.5
Visual: white crusts on soil
2. SODIC SOIL: They have much high pH and ESP than saline soils. These soils are formally called black alkaline soil, because the high pH dissolve organic matter which is deposited on the surface along with the excess salts (primary sodium).

However, the soil solution of sodic soils are dominated by sodium which tends to disperse soil particles leading to negative impacts on soil physical structure and reduced water infiltration. The physicochemical characteristics of sodic soils include:
Electrical Conductivity: <4 ds m-1
Exchangeable Sodium Percentage: >15%³
Sodium Adsorption Ratio: >13%
pH: >8.5
Visual: Dispersed, crusts, structure compromised, can be very dark in color
3. SALINE/SODIC SOIL: These soils are mixtures of salt content and high ESP. When leached, the pH tend to increase, and the soil becomes SODIC. The physicochemical characteristics include:
Electrical Conductivity: > 4 4 ds m-1
Exchangeable Sodium Percentage: >15%
Sodium Adsorption Ratio: >15%
pH: <8.5
Visual: Not dispersed, okay structure, not usually dark in color

CAUSES OF SOIL SALINITY
Soil salinity occurs when soluble salts are retained in the soil. It can occur naturally or due to farming practices. Although some soils are initially saline due to low salt dissolution and removal. Some other causes include;
1. When annual precipitation is insufficient to leach ions from the soil profile, salts accumulate in the soil resulting in soil salinity.
2. When water table is at a shallow depth and moisture is drawn to the soil surface by capillarity bring with it dissolved salts which are left behind as the water evaporates.
3. Flooding by sea water
4. Inappropriate application of fertilizers when excess nitrification accelerates soil salinization.
5. Breezes in the coastal areas that blow salty air masses to the nearby territories.
6. Irrigation with salt-rich water, which amplifies salt content in earths.
7. Poor drainage or waterlogging when salts are not washed due to a lack of water transportation.
Soil salinity result in micro elements accumulation to toxic proportion . Boron concentration becomes toxic at 1micro gram per gram. Generally, plants cannot tolerate a salt concentration over 0.5%.
8. Poor water quality and irrigation practices also contribute to the salinization
9. Soil salinity result from parent materials. Salts in the soil occur as ions (electrically charged forms of atoms or compounds). Ions are released from weathering minerals in the soil.
METHODS USED TO CORRECT SALINE AND SODIC SOIL
Saline and sodic soil conditions can be corrected by chemical and physical methods. These methods seek to replace excess sodium with Calcium and remove it by leaching with high quality irrigation water. In many cases, drainage is required to facilitate leaching. For soil with high pH and containing CaCO3, the addition of sulphuric acids or elemental sulfur may be used to dissolve the lime and free Ca to displace the Na. In other situations, gypsum is recommended.
Salt tolerant crops can also be planted to reclaim salt affected soils. Such crops include cotton, bamuda grass etc.
INDICATORS OF SOIL SALINITY
Salinization can be determined visually by analyzing the soil surface, speed of water infiltration, and vegetation state. As soils become saline, visual changes are noticed. The more the soil salinity, more severe the changes. For example, slight whitening on the surface changes into distinct salt crystals. Apart from visual changes, indirect indicators of extra salt concentration can be sighted. Suh include; poorer water quality or animal behavior when livestock refuses to drink water due to its salty taste.
SALINIZATION -RELATED INDICATORS IN VEGETATION
a) PLANT WITHERING ; Plants uses osmotic process to absorb water. When soil is saline, plants will not be able to absorb water from the soil but release water into the root zones. This causes the plant to wither.
b) CROP LOSS ; When plants wither due to osmotic stress caused by strong soil salinity, they die.
c) REDUCTION OF BIODIVERSITY ; Majority of plants can survive on non saline soils. Only few plants can survive on saline soils. Therefore, a reduction in plant biodiversity occurs on saline soils
d) APPEARANCE OF SALT – TOLERANT PLANTS IN THE AREA AND THEIR FURTHER DOMINANCE. Only plants that are salt tolerant will survive on saline soils. Thus, they dominate areas with salinity problems

SOIL SALINITY MEASUREMENT
Soil salinity can be estimated visually. Other methods used to measure soil salinity include assessing the soil ‘s electric conductivity with specific devices. With a rise of salt concentration in the solution, its conductivity rises, too. And calculating the exchangeable sodium percentage or sodium absorption ratio using the above formular. Also, salinity sensors are used for monitoring surface soil salinity in affected areas.
ADVERSE EFFECTS OF SALINIZATION
Soil salinity affects crop production and water quality, induces the risk of floods and soil erosion, and decreases biodiversity.

1. CROP PRODUCTION
Salinity decreases the agricultural production of most crops. It affect almost or all aspects of plant development from germination, vegetative growth to reproductive development. It also affects soil physicochemical properties, and ecological balance of an area.The impacts of salinity include—low agricultural productivity, low economic returns and soil erosions
For plants to uptake nutrients from the soil, water is needed. Water saturation in plants depends on the level of salts in groundwater and the plant itself. Plants absorbed water through the process of osmosis (The water flows from less salt-concentrated areas to more concentrated areas). If the soil is highly concentrated with salt, water will be released by the plant into the soil solution and their will be no nutrient uptake. Such plants will suffer from osmotic stress if the water release gets to a threshold. Thus, the plant will wilt and die just like in drought stress conditions. Therefore, Soil salinity imposes ion toxicity, osmotic stress, nutrient (N, Ca, K, P, Fe, Zn) deficiency and oxidative stress on plants, and thus limits water uptake from soil.
Another soil salinity effect on agriculture is ionic stress due to harmful ions in soil salts. Soil salt contain chloride or sodium ions. And with boron, toxic effects on plants occurs. Excessive accumulation of sodium in cell walls can rapidly lead to osmotic stress and cell death. Apart from their toxic impact, positively charged ions like sodium do limit the uptake of other positively charged ions vital for crop growth (particularly potassium and calcium)
2. FLOOD RISK
Water table rises due to salinization and it reduces the soil’s ability for water infiltration. When water table is shallow, there is high potential of flooding occurring. Soils under this condition cannot absorb rainwater, thus runoff occurs which result in flooding. Also, when their is heavy rainfall or river flooding, soils will not be able to absorb the high amount of the water flows. Thus, result in flooding. This then result in damage to constructions like roads, fences, and dams, damage farmlands and wetlands, increase sedimentation, and contaminate aquatic bodies.
3. WATER QUALITY
High amounts of salt in water adversely affects the life of the plant and aquatic species. When water flows between rocks comprising soluble minerals, the water carries along with it dissolved salts from the rock. These salts flows over the soil surface causing the soil to become saline. Also, the salty water may be used for irrigation, or industrial usage. Water that has salt content in the range of 10,000 ppm to 35,000 ppm are classified as highly saline water.

This salt can also penetrate to fresh water bodies causing water salinization. This deteriorates drinking water for humans and domestic animals; and irrigation water and adds to further salinization of drylands, contaminates farmlands,
deprives river species of their natural habitats. It indirectly causes Health problems such as
excessive salts in the body putting a great load on the kidney impairing its working order and sometimes causing total kidney failure. Ineffective kidneys lead to heart and high blood pressure problems.
In the case of plants, water with high concentration of chloride ions result in plant poisoning and its eventual death. Plant growth is hampered because of dehydration and interference with nitrogen uptake. When vegetation on land becomes dehydrated for a long period, all vegetation would wilt and die, living the soil bare and thus, leads to soil erosion.
In addition, High levels of salts may affect the taste of drinking water. Chloride in particular has a low taste threshold. Sodium and magnesium sulfate levels in drinking water may produce a laxative effect and reduce the suitability of a water supply for grazing animals.
4. BIODIVERSITY
Salinity reduces soil respiration, enzyme activity, soil microbial biomass, plant population and bacterial growth rate. Non-saline soils do not affect crops , animals or micro organism, while few of these organisms can survive and tolerate strongly saline soils . For example, halophytes plants can survive on saline soils. Crops need sufficient amount of organic matter to supply their nutrient needs. The decomposition of organic resiues is carried out by microbs. Salinity also determines the regulation of soil microbial activity and community. Some studies have reported that salinity is the most important factor that determines the distribution patterns of microbes particularly bacterial and fungal communities .Salinity decreases bacterial and fungal growth. Bacterial and fungi community decreased with increasing soil salinity. Therefore, rate of decomposition of organic matter reduces with increase in salinity. Salinization also reducesr flora diversity. Thus, causes a reduction of fauna population. Salinization reduces biodiversity in rivers or fresh-water lakes, shortening aquatic populations solely to salt-tolerant species.
4. SOIL EROSION
Soil salinization is a serious problem that causes soil degradation including soil erosion. . Salinity causes continuous wetness of land surface and a lack of surface cover due to poor plant growing conditions. These make lands highly prone to erosion. Most vegetation cannot survive on strongly saline soils as they are subjected to osmotic stress. This result in land surface without vegetation cover. Thus, the soil is prone to water or wind erosion.
MANAGEMENT AND PREVENTION OF SOIL SALINITY
Soil salinity prevention should be bases on avoidance of salt from accumulating on the farmland and in water bodies. Some of the methods used to prevent soil Salinization include:
1. Water to use for irrigation should contain less amount of salt, use of harvested rain water for irrigation
2. Application of organic matter and manure to soil to keep it moisture rather than irrigation. And to increase microbial population. The organic matter also improve soil structure, drainage, and moisture-holding capacity of the soil.
3.Use cover crops or mulch to protect the ground surface from erosion and runoff. And also to prevent evaporation and subsequent build-up of salt in the soil.
4. mixing topsoil with residues of harvested plants etc.
5. Planting trees and crops that are salt-tolerant,
6. Construct drainages to surround the salt affected areas so that the salt is not washed to unaffected areas and water bodies.
7. Plant sensitive plants uphill, on berms, or in raised beds where salty water will not drain toward or accumulate.
8. Use windbreaks (fences and buildings) to intercept aerial salt drift from reaching sensitive plants.
9. Fertilize only when a soil test or plant symptom indicates that fertilizer is needed, and then only at rates recommended by soil analyses and fertilizer labels
HOW TO REDUCE SOIL SALINITY
There are several techniques used to tackle salinization and improve agricultural productivity. Some of which are: 1. Increase field drainage for better flushing (to remove salts from the ground surface).
2. Plant salt-tolerant crops to manage economic risks and to ensure land cover.
3. Planting of tolerant cover crops
4. Field rinsing: This requires lot of water to dissolve the salt and wash it down to lower horizons
5. Deep ploughing that helps break down the compacted soil layer created by the weight of agricultural machinery or by the natural formation of claypan layer. This will allow water to pass freely into the lower layers and thus wash away the salts that have accumulated overtime
6. Remove salt crystals from the surface mechanically.
7. Application of chemical amendments such as gypsum or sulfuric acid.
8. Reduce evaporation with mulch, cover crops or crop residue.
9. Mulches can be applied to crops to save water
10. Apply fertilizers rationally, as an overuse of certain chemicals promote salinization.
HALOPHYLIC PLANTS
halophyte is a salt-tolerant plant that grows in soil or waters of high salinity. Some of these plants include:

Anemopsis californica — – lizard tail
Atriplex—-saltbush or orache
Attalea speciosa—–babassu
Panicum virgatum—–switchgrass
Salicornia bigelovii—-dwarf glasswort or pickleweed
Spartina alterniflora—-smooth cordgrass
Tetragonia tetragonoides—–warrigal greens, kōkihi, sea spinach
Dunaliella—-a green alga
Sesuvium portulacastrum—-sea purslane or shoreline purslane
Suaeda—–Seep-weeds
Halimione portulacoides—–sea purslane
Sarcocornia fruticosa—–saltworts
Phoenix dactylifera—- date palm
Hordeum vulgare—– barley
