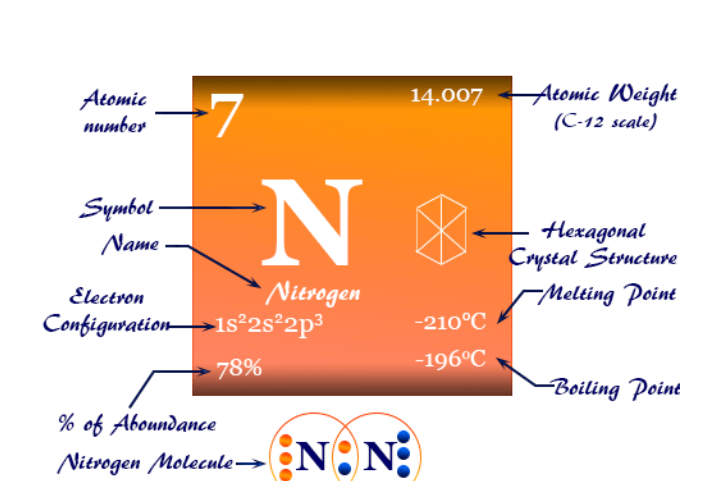
Nitrogen is an essential nutrient used in relatively large amount (macronutrient) by all living things. It has a dramatic effect on plant growth and It is critically important to plants because it is a fundamental part of the chlorophyll molecule and it is essential in the formation of amino acids and proteins. Plants obtain their nitrogen as inorganic nitrate and ammonium ions in the soil solution. The nitrite ion form cannot be uptake by plants because it is unstable and toxic in high amount.
Nitrogen is not a natural constituent of rocks or minerals, rather, its natural state is as nitrogen gas in the atmosphere. 78% of the air we breath is nitrogen by volume. This atmospheric nitrogen is very non reactive and it is not plant available.
In soil, Ammonium ions can bind to the soil colloids because it is positively charged cation while nitrate ions cannot bind to the soil colloids because they have negative charges.

In plants, ammonium can be used directly but nitrate is first transformed to the ammonium form using energy derived from photosynthesis. The ammonium ions are combined with carbon ions to form glutamic acid which is then used by plants to produce a large number of amino acids. The amino acids are joined together to form proteins. The proteins formed act primarily to control plant growth processes through enzymatic action.
A good supply of nitrogen is associated with vigorous growth and a deep green colour. Plants deficient in nitrogen becomes stunted, produce smaller fruits and flowers and yellow (chlorotic) in appearance. Since plants can remobilize nitrogen from older tissues to provide nitrogen to younger tissues, Chlorosis usually appear on the lower leaves first while the upper leaves remain green.
EFFECT OF LOW NITROGEN ON PLANTS
1. Too little amount of nitrogen result in inability of plants to thrive, leading to low crop yields.
2. When plants do not get enough nitrogen, they are unable to produce amino acids (substances that contain nitrogen and hydrogen and make up many of living cells, muscles and tissue). Without amino acids, plants cannot make the special proteins that the plant cells need to grow. Therefore, without enough nitrogen, plant growth is affected negatively.
3. Deficiency of nitrogen result in yellow colouration (Chlorosis) of older leaves on plants while younger leaves remain green

4. Insufficient nitrogen causes stunted growth in plants
5. Insufficient or deficiency of nitrogen causes smaller fruits development in perennial crops.
EFFECT OF EXCESS NITROGEN ON PLANTS
1. Excess nitrogen may cause plants to remain in a vegetative growth stage and delay initiation of flowering or fruiting, resulting in lowered yields of some crops.
2. Excess nitrogen can also encourage tender, succulent plant growth that may be more susceptible to certain plant diseases. An example of this is glume blotch (Septoria nodorum) which can infect the heads of wheat and cause severe yield reduction.
3. Plants with excess nitrogen may also be more susceptible to lodging and breakage than plants without excess nitrogen and very often are more likely to be damaged by freezing temperature.
4. Too much nitrogen can be toxic to plants, and can also harm our environment. It can hurt plants and animals, and pollute our aquatic systems
5. Excess nitrogen can also leach—or drain—from the soil into underground water sources, or it can enter aquatic systems as above ground runoff. This excess nitrogen can build up, leading to a process called eutrophication
6. With too much nitrogen, plants produce excess biomass, or organic matter, such as stalks and leaves, but not enough root structure. In extreme cases, plants with very high levels of nitrogen absorbed from soils can poison farm animals that eat them.
Available Nitrogen under natural conditions are quickly used up by organisms. Therefore, nitrogen is said to be a limiting growth factor in nature especially for crop production.
There is a balance between atmospheric nitrogen and soil nitrogen. Nitrogen fixation by microbs, return of nitrates and ammonium by precipitation are balanced by losses to crop removal, leaching and volatiliztion. Man has now devices a way of converting atmospheric nitrogen into chemical unstable nitrogen compounds used for fertilizers and other products.
FORMS OF NITROGEN
There are two forms of nitrogen. Organic and inorganic nitrogen
1. ORGANIC NITROGEN: Organic sources of nitrogen include proteins, amino acids, amino sugars, and other complexes. Soil nitrogen is in organic form.
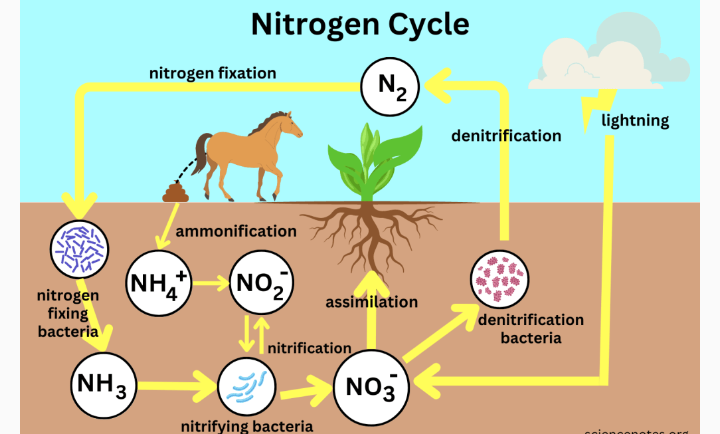
2. INORGANIC NITROGEN : Inorganic nitrogen compounds are unstable compounds. Such nitrogen are constantly returned to the atmosphere in gaseous form. The conversion of nitrogen to nitrogen compounds and from nitrogen compounds back to atmospheric nitrogen is called NITROGEN CYCLE. From findings, it is discovered that it takes 44 to 220 million years for all nitrogen to pass through the cycle.
INORGANIC SOURCES OF NITROGEN
N2 : from Inert nitrogen gas found in the atmosphere
NOx: Nitrous oxide, is found in the atmosphere and it is a component of automobile exhaust and industrial process
NH3: Ammonia, it is a volatile gas and it is often loss from soil applied ammonium fertilizers into the atmosphere
NH4+: Ammonium, it is a positively charged cation found in the soil
NO2-: Nitrite, it is a negatively charged anion found in the soil
NO3-: Nitrate, it is a negatively charged anion found in the soil and at times in the atmosphere.
Plants absorb most of their nitrogen in the ammonium(NH4+) or nitrate (NO3-) form. The availability of these two forms of inorganic nitrogen for plant uptake depend on the liberation of the inorganic nitrogen from organic sources or the application of fertilizers which contain either NH4+ or NO3-
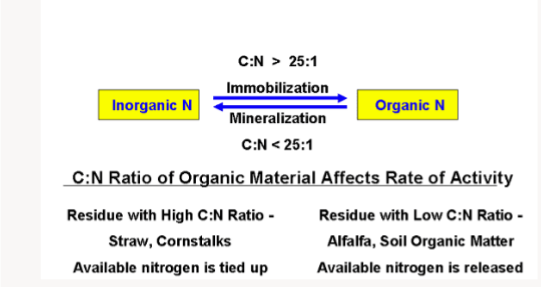
IMMOBILIZATION AND MINERALIZATION OF NITROGEN
Soil nitrogen is a dynamic compound which usually shift position between organic and inorganic forms.
Immobilization of nitrogen means the absorption of plant availabile nitrogen forms ( NH4+ or NO3-) by plants and microbs and their transformation into amino acids and proteins. The amino acid and protein forms are not available for plant and microbial uptake but immobilised in plants and microbs tissues.
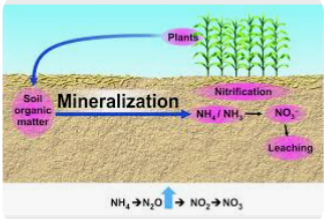
When these organisms die, the organic nitrogen compound in their tissues will be decomposed by microbs like bacteria, fungi and other organisms to liberate plant available inorganic nitrogen forms ( NH4+, NO3- and NO2-). The process of decomposition and liberation of these three inorganic nitrogen forms ( NH4+, NO3- and NO2-) from plant tissues is known as mineralization. Mineralization happens when microbes act on organic material, such as animal manure or decomposing plant or animal material and begin to convert it to a form of nitrogen that can be used by plants.
In mineralization, mineral forms of nitrogen are liberated. Both mineralization and immobilization processes are processes that occur continuously in the soil and they create a balance within the soil( that is, as more nitrogen is being mineralized in the soil through organic matter decomposition, so is immobilization of nitrogen through absorption by plants occurring). This balance can also be disrupted. This can occur when organic residues with high C:N ratio are incorporated into the soil system. This type of organic residues are difficult to decompose because of their high carbon content. Such materials include straws, dry leaves, maize stalk, and saw dust etc. Some of the compounds in these materials that make them difficult to decompose include lignin, cellulose, fats, and resins. When these crop residues are incorporated into the soil, micro organisms begin to decompose them. The microbs utilized the carbon components as an energy source and the available nitrogen to form proteins used to build their body tissues. This brings about a decrease in the soil nitrogen level because the micro organisms will compete with themselves for the little quantity of nitrogen in the plant tissues, they also compete for plant nutrients. CO2 is liberated from the process into the atmosphere. When they finish decomposing the organic residues, no more food source will be available, therefore, the microorganisms begin to die. The microorganisms body begin to decompose and the protein in their body mineralize to liberate plant available NH4+ and NO3-.
Organic residues with low C:N ratio easily decompose because of the low carbon content in them. Note that the more immature a plant material, the lower the C:N ratio. The decomposition rate of such immature plant materials are high and they contribute to the overall soil nitrogen content. Such plants contain easily decomposable compounds like sugars, proteins, starch and hemicellulose.
STEPS IN MINERALIZATION PROCESS
Mineralization process involves the following sub- peocesses:
Amminization, ammonification and nitrification sub processes. These nitrogen mineralization processes involve the use of enzymes, which include: deaminases, O-glycosidases, and acetyl hydrolases
1. AMMINIZATION SUB-PROCESS
Amminization and ammonification sub processes are brought about by heterotrophic microorganisms which include bacteria and fungi. These organisms are involved in all steps in organic matter decomposition. The first step of mineralization is called amminization and it is also the last step in the decomposition of organic materials. Proteins are hydrolysed to form amines, amides and amino acids.
That is,
Protein= R-NH2 + CO2 + energy + other products.
2. AMMONIFICATION SUB-PROCESSES
The amines and amino acids produced as end products during the Amminization process are broken down by other soil heterotrophs to produce ammonical compounds (ammonium). This process is called ammonification. It is the second step of mineralization and represented as
R-NH2 + HOH= NH3 + R-OH +energy
This process takes place in both aerobic and anaerobic conditions, but proceeds rapidly in the oxygen-rich layers of the soil. The ammonia produced is used for
a. It is converted to nitrites and nitrate in the nitrification process
b. It is used directly by plants
c. It is used by soil microorganisms
d. It is held up by certain clay soil
3. NITRIFICATION SUB- PROCESS
Nitrification is the conversion of ammonium through oxidation process to Nitrate. Energy
released during the process is used up by the soil microorganisms.
Nitrification process is carried out by autotrophic soil microorganisms.
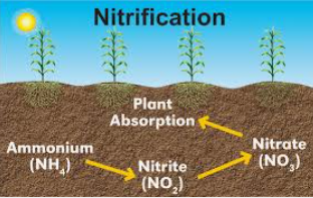
Nitrification process is in two steps
1. Ammonium is converted to nitrites by obligate autotrophic soil microorganisms called NITROSOMONAS.
2NH4+ + 3O2= 2NO2- + 2H2O + 4H+
Nitrification inhibitors, such as nitrapyrin (N-ServeR) or dicyandiamide (DCD), interfere with the function of the bacteria in this first stage of nitrification, blocking ammonium conversion to leachable nitrate.
2. Nitrite produced in the first step is converted to Nitrate by another obligate autotrophic soil microorganisms called NITROBACTER.
2NO2- +2O2= 2NO3-
SUMMARY OF NITRIFICATION STEPS
Nitrosomonas Nitrobacter
Organic Nitrogen ———> Nitrite ———> Nitrate
The NO3 in soil is highly Mobile and can be lost to leaching. It is the available form plants can uptake nitrogen from the soil and it can cause surface and underground water pollution if it gets to the water body.
The above processes takes place under aerobic soil conditions. Under anaerobic soil condition when water had filled soil pore spaces and no pore spaces, mineralization processes is slowed down or prevented from occurring. The mineralization process also depend on soil temperature, soil moisture, soil pH, tillage system, cropping systems C/N ratio, soil conditions and available plant nutrients in the soil.
HOW NITRIFICATION CAUSES ACIDIFICATION
1. During Nitrification, two H+ cations are liberated which reduces soil pH.
2. Any N in ammonium form will decrease soil pH
3. Continuous use of fertilizers like ammonium sulfate, ammonium nitrates and urea will decrease soil pH.
FACTORS THAT INFLUENCE NITRIFICATION AND MINERALIZATION PROCESSES
Mineralization and nitrification are influenced by environmental factors that affect biological activity such as temperature, moisture, aeration, oxygen and pH etc.
a. TEMPERATURE : At low temperatures, Nitrification process is slow and ceases once the temperature declines below freezing. While at high temperature, Nitrification rate increases until bacterial viability is reduced (around 95oF to 100oF), and then a decline of nitrification begins to occur with further increase in temperature. b. MOISTURE AND OXYGEN :Moisture and oxygen are necessary for microbial function in both the mineralization and nitrification processes. Excessive moisture limits oxygen availability in the soil, reducing mineralization and nitrification rates. Mineralization cannot take place under anaerobic soil conditions.
c. pH LEVEL: Rates of mineralization and nitrification processes occur rapidly at pH levels near 7, and decline as soils become excessively acid or alkaline
DENITRIFICATION
This is the reduction of nitrates to N2 and N2O. It takes place under anaerobic condition when the soil is waterlogged. Denitrification losses vary with degree of soil saturation, duration of saturation, organic matter content, and soil pH.
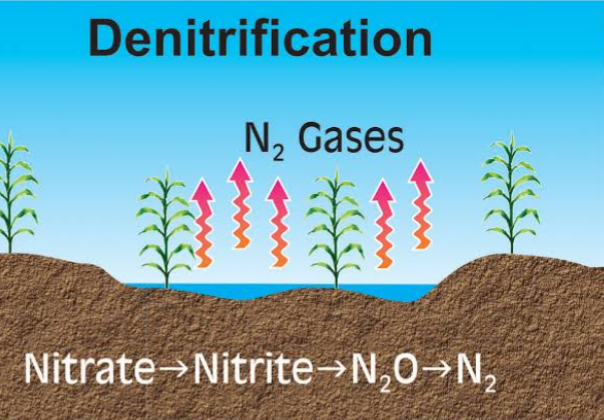
To prevent nitrogen losses in Nitrification process, adequate soil aeration, reduction in soil waterlogging and maintaining soil pH between 5.5 to 7 should be carried out.
NO3– ——> NO2——>NO ——> N2O ——> N2
NITROGEN FIXATION
Nitrogen can be fixed into the soil either by natural or artificial processes. In both ways, N gas is transformed into plant available forms ( NH4+ and NO3-). The following are ways N2 are fixed to the soil
1. BIOLOGICAL FIXATION
Biological fixation may be symbiotic or nonsymbiotic nitrogen fixation
a. SYMBIOTIC NITROGEN FIXATION: This is when a host plant houses microorganisms that fix nitrogen into the soil. Both host plants and microorganisms benefits from one another in this relationship. Example can be seen in rhizobium bacteria which live in the root nodules of legumenous plants like soybeans, cowpea, sesame, bambaranut, groundnut etc. The bacteria infect the legume plant roots and form nodules. The bacteria fix atmospheric N2 into the soil and make it available for plant uptake. The legume Inturn provide carbohydrates to the bacteria which supply the bacteria ‘s energy need.
b. NON SYMBIOTIC NITROGEN FIXATION
Non symbiotic nitrogen fixation is carried out by free living bacteria and blue green algae. The amount of N2 fixed by non symbiotic organisms are lesser than the amount fixed by symbiotic organisms.
2. FIXATION OF NITROGEN BY LIGHTNING
Nitrogen may be fixed into the soil by electrical discharge of lightning in the atmosphere. The heat from lightning can form NO3-N which dissolve in rain water and gets into the soil.
Industrial pollution may also contribute to the N level that dissolves in rain water.
3. SYNTHETIC OR INDUSTRIAL PROCESSES OF NITROGEN FIXATION
In industrial processes of nitrogen fixation, ammonia ( NH3) is synthesised from nitrogen (N) and hydrogen (H).
N2 + 3H2= 2NH3.
The hydrogen is obtained from natural gas and nitrogen from the atmosphere. The ammonia serves as a raw material for many other materials that contain nitrogen such as ammonium sulfate, ammonium nitrate, sodium nitrate, and ammonium phosphate etc. It should be noted that all commercially manufactured fertilizers come from ammonia formed from atmospheric nitrogen.
LOSSES OF NITROGEN
Nitrogen can be lost from agricultural soils through the following processes. Volatilization, leaching, crop removal, and runoff.
1. VOLATILIZATION
a. This is the gaseous loss of ammonia from ammonium fertilizers. When a soil is alkaline (that is, high pH) or acidic soils that had just be neutralized using limestone, application of ammonium fertilizers like ammonium sulfate, ammonium nitrates, or urea, the ammonium (NH4) may be converted to ammonia (NH3) which may be lost to the atmosphere
SOLUTION: In alkaline soil, the fertilizers should be incorporated into the soil.
b. When urea is applied on the soil surface with adequate moisture and temperature, the urea will naturally volatilize.
SOLUTION: The urea should be incorporated into the soil or banded into the soil.
Also, immediately after application of the urea, the field should be irrigated to dissolve the urea making it sink into the ground
c. ANHYDROUS AMMONIA: This is a gaseous form of nitrogen which when handled improperly can volatilize.
SOLUTION: To prevent losses, the material must be incorporated into the soil at sufficient depth. Also when applying, soil must be moist and not saturated.
The potential for ammonia volatilization is influenced by soil moisture, temperature, soil pH, soil buffering capacity, urease activity, residue cover, precipitation, wind and other factors.
2. LEACHING: For leaching to occur, nitrogen must be in a water soluble and mobile form for the nitrogen to be transported through the soil. Leaching occurs when inorganic nitrogen form like nitrites and nitrates are solubulized and carried with water down the soil horizons. The nitrites and nitrates can pollute surface and underground water.
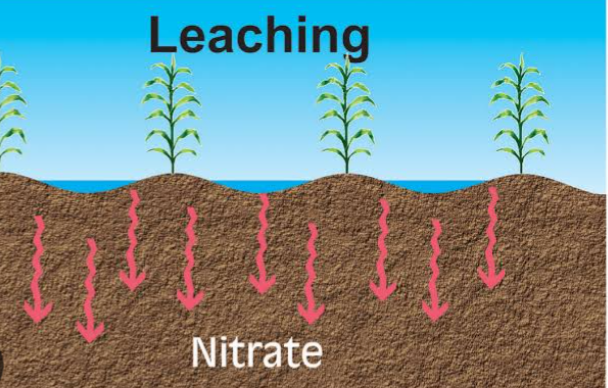
The rate of nitrate movement down the horizons depends on a variety of factors such as soil texture, precipitation and irrigation amounts, and crop uptake of water and amount of nitrate in the soil.
3. RUNOFF: Runoff can occur when inorganic nitrogen form like nitrites and nitrates are carried by with water along the soil surface to another location. Or nitrogen ours fertilizers applied are washed away on soil surface. The nitrogen forms can be washed into nearby water bodies thereby polluting it. It can cause heavy growth of algae and water plants.
4. CROP REMOVAL: Many crop materials contain some amount of nitrogen uptake from the soil. These crop materials such as grains, leaves, stems, and forages etc can be harvested and exported from the farm. This means that some quantity of nitrogen had being removed from the farm. However, if crop residues and manures are returned back to the field, then the nitrogen in these materials can be recycled and returned back to the field for future usage.
EUTROPHICATION.
Eutrophication is a situation when excessive nitrogen enriches the water, causing excessive growth of plants and algae. Too much nitrogen can even cause a water body to turn bright green or other colour due to a “bloom” of large biomas of smelly algae called phytoplankton. When the phytoplankton or algae die, they sink to the bottom of the water body where microbes in the water decomposes them with large consumption of oxygen in the process. The overconsumption of oxygen leads to hypoxic conditions (conditions in which the amount of dissolved oxygen is low) in the water. This can lead to what is called a “dead zone”. Dead zones do not have enough oxygen to support most aquatic life form.The hypoxic conditions of the dead zone of the water body lead to the suffocation and eventual death of larger life forms such as fish. Dead zones can occur in water bodies like freshwater, lakes and rivers where runoff empty the agricultural nutrients it carried.

