Heavy Metals are naturally occurring metals in the environment which are subdivisions of elements characterized with metallic properties. They include transition metals, some lanthanide, metalloids and actinides. There are about 92 elements exiting in nature of which only 30 are metalloids. They include Be, B, Li, Co, Cu, Zn, Al, Ti, V, Cr, Mn, Ni, As, Se, Sr, Mo, Pd, Ag, Cd, Te, Sb, Ba, Cs, Au, W, Pt, Pb, Au, Hg, and Bi.
Heavy metals are vital for survival, but they may become hazardous when they accumulate in organisms. They are nonbiodegradable and have a lengthy half-life. Their toxicity is a major environmental concern with its adverse effect on human health, crop production and the ecosystem. They stay longer in the atmosphere causing air pollution.
Heavy Metals have high density and atomic weight. They are elements which have specific weight of more than 5 g cm3 . And any metals that are at least 5 times denser than water are also referred to as Heavy Metals. They are found in the biosphere, such as in water, soils, and rocks, and are also released into the surroundings from anthropogenic resources, mostly through commercial and industrial wastes products. They can be classified as essential or non-essential trace elements. When in excess, result in physiological or morphological abnormalities or genetic mutations, such as slowing or stopping growth or causing mutations.
Plant nutrients are macro nutrients and micronutrients. These nutrient elements especially the micronutrients can either be refer to as heavy metal or not, causing pollution to the environment.
The essential heavy metals which are micronutrients ( that is, they are required in small quantities for the normal growth of plants and/or animals) include (Mo, Mn Cu, Ni, Fe, Zn) or non-essential metals (Cd, Ni, As, Hg, Pb), when in excess are termed heavy metals. For example, Copper (Cu), manganese (Mn), molybdenum (Mo), nickel (Ni) and zinc (Zn) can become heavy metals eventhough they are essential for higher plants. For animals and humans, chromium (Cr), copper (Cu) , cobalt (Co), Mn, Mo, selenium (Se), vanadium (V) and zinc ( Zn) are the micronutrient heavy metal(loid)s. Iron (Fe), not usually considered a heavy metal is essential for both plants and animals. Several other elements, including arsenic (As), cadmium (Cd), lead (Pb) and tin (Sn) may possibly have an essential role at very low concentrations. But at high concentration, they are heavy metals.
Heavy metal pollutants can restrict the growth of plants, affecting the plant system, seed germination, production, physiological, biochemical, and genetic elements.
They can be naturally introduced into the environment through processes such as wind, erosion of soil, forest fires, volcanic eruptions, biogenic processes, and the release of marine salt. Anthropogenic contamination of the environment by heavy metals can occur through various means, such as mining operations, the utilization of fertilizers, herbicides, and pesticides, and the irrigation of agricultural land with untreated sewage and industrial effluent.
Heavy metals can enter the human body through various routes such as absorption, skin contact and inhalation
SOURCES OF HEAVY METALS
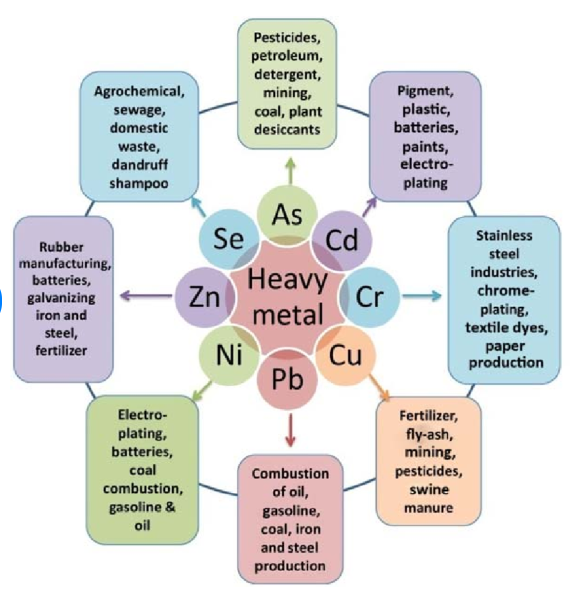
Most heavy metals occur naturally, but a few are derived from anthropogenic sources. The most common ones which can pollute the environment are Arsenic, cadmium, chromium, copper, nickel, lead and mercury. They are essential to life but can become toxic through accumulation in organisms. Mercury, lead and cadmium are of greatest concern because of their ability to travel long distances in the atmosphere.
There are majorly two sources of heavy metals. They include anthropogenic sources and natural source.
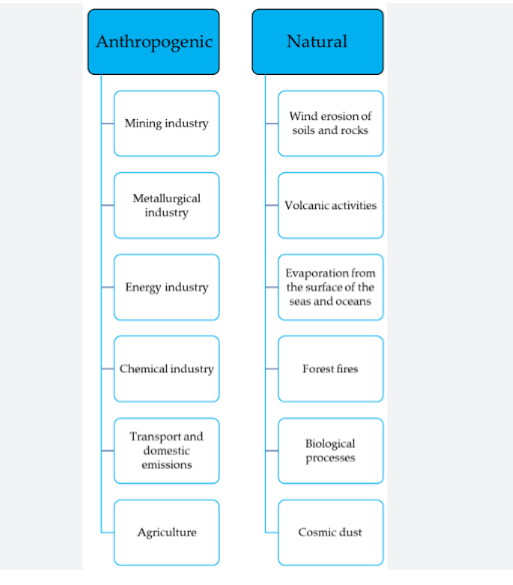
1. ANTHROPOGENIC SOURCES OF HEAVY METALS : Human activities such as industrial operations, mining, irrigation of crop fields with industrial water, and agricultural practices contribute significantly to heavy metal pollution,
Research has shown that agricultural and industrial activities that do not have a single identifiable source, known as non-point source pollution, are major contributors to the presence of cadmium, nickel, lead, zinc, arsenic, and mercury in the environment.
2. NATURAL SOURCES OF HEAVY METALS :
Natural sources such as volcanic eruptions, weathering of rocks, biogenic processes, and wildfires also contribute to the introduction of heavy metals into the environment
CHARACTERISTICS OF HEAVY METALS
Other characteristics of Heavy metals are;
1. They have high atomic mass and toxicity to living organisms.
2. Most heavy metals cause environmental and atmospheric pollution, and may be lethal to humans.
3. Heavy metals can become strongly toxic by mixing with different environmental elements, such as water, soil, and air,
4. Humans and other living organisms can be exposed to them through the food they consume.
5. Sources of heavy metals include mining, industrial production (foundries, smelters, oil refineries, petrochemical plants, pesticide production, chemical industry), untreated sewage sludge and diffuse sources such as metal piping, traffic and combustion by-products from coal-burning power stations
6. Heavy metals, unlike organic pollutants, are non- biodegradable and tend to accumulate in living organisms when they are released into the environment,
MITIGATION AND CHEMICAL TRANSFORMATION OF HEAVY METALS
Migration and transportation of heavy metals can be caused by several factors such as bacterial activities, temperature and physicochemical Conditions of the environment
Bacteria degradation of polluted sites do bring about chemical transformations of heavy metals though the following processes ; oxidation, reduction, methylation, and demethylation which are sometimes byproducts of normal metabolism and contribute no known advantage on the bacteria. Atimes, the transformations constitute a mechanism of resistance. Many species of bacteria have genes that control resistances to specific toxic heavy metals. These resistances often are determined by extrachromosomal DNA molecules (plasmids) in their body system. The same mechanisms of resistance occur in bacteria that transform heavy metal in soil, water, industrial waste, and clinical sources. For example, in mercury polluted sites, mercury and organomercurial are converted through enzymatic detoxification into volatile substances (methane, ethane, metallic HgO) which are rapidly lost from the environment
In an investigation on migration and transformation of heavy metals using pyrolysis method, transformation of heavy metals using heat treatment was carried out in predried dyeing sludge (PDS) char samples prepared on a fixed bed in a pure nitrogen atmosphere. It was discovered that hydrochloric acid soluble Cu was transformed to the insoluble speciation at 400–500 °C. A small amount of Hg transforms from the hydrochloric acid soluble speciation to the gaseous speciation. Ni was observed to transform from the hydrochloric acid soluble speciation to the insoluble speciation below 700 °C, while it transforms from the insoluble speciation to the hydrochloric acid soluble speciation above 700 °C.
LEACHING PROCESS OF HEAVY METALS
Acid rain can lead to acidification of surface waters and soils. Acid rain is caused by a chemical reaction that begins when compounds like sulfur dioxide and nitrogen oxides are released into the air, they mix and react with water, oxygen, and other chemicals to form more acidic pollutants, known as acid rain. The SO2 and NOx are emitted to the atmosphere, during fossil-fuel combustion. Acid rain is able to enhance metal mobilization in soil ecosystems. The H+ ion in the acidic water displaces cations from their binding sites, thereby reduces the cation exchange capacity (CEC), and increases the concentrations of the cations in the soil-water system. The negatively charged sulfate and nitrate ions in the acid rain act as “counter-ions”, which allow cations to be leached from the soil. Through a series of chemical reactions, cations such as K+, Na+, Ca2+ and Mg2+ are leached out and become unavailable to plants as nutrients. Likewise, toxic ions, such as Cu, Pb and Cd, usually bound to the negatively charged surface of soil particles and can be displaced by H+ ion too.
The leaching process of heavy metals is actually migration of metal ions which is related to adsorption–desorption, complexation–dissociation, and precipitation–dissolution reactions. When heavy metals are leached from the soil, groundwater may be polluted.
MODE OF DEPOSITION OF HEAVY METALS
a. Heavy metals are poorly water-soluble, often migrate as small suspended particles. Industries and natural resource exploitation emit particules of air pollutants which causes soil contamination. These atmospheric particles, usually settles by gravity and get deposited on the ground through both dry and wet depositions. b. Beside water bodies like river banks, heavy metals are enriched in sediments deposited at the banks . Many factors affect the Heavy metals deposition process. Such factors which are physicochemical properties of the environmental sites include, particle size, mineral composition, vegetation cover and organic matter content, affect the migration and deposition of heavy metals.
Heavy Metals can be deposited and removed in two ways. Heavy metals present in air can be deposited in the soil through dry and wet deposition. Such depositions are associated with the periodic fluctuations in rainfall periods.
1. DRY DEPOSITION: Dry deposition of particles occurs by direct impact and gravitational settling on land or water surfaces.
2.WET DEPOSITION: Wet deposition refers to the process in which particulate and gaseous pollutants in the atmosphere dissolves in weather processes such as wet deposition, snowfall, hail, and fog, and settle on surfaces. Wet deposition is also one of the main ways these air pollutants are moved from the atmosphere. The particulates inform of aerosols and gases are dissolved or suspended in water droplets or ice crystals and get settled on leaves, branches, roofs and other surfaces which are later wash down by rainwater to the soil or groundwater.
Wet deposition is common in coastal region.
TREATMENT METHODS FOR REMOVING AND ERADICATION OF HEAVY METALS
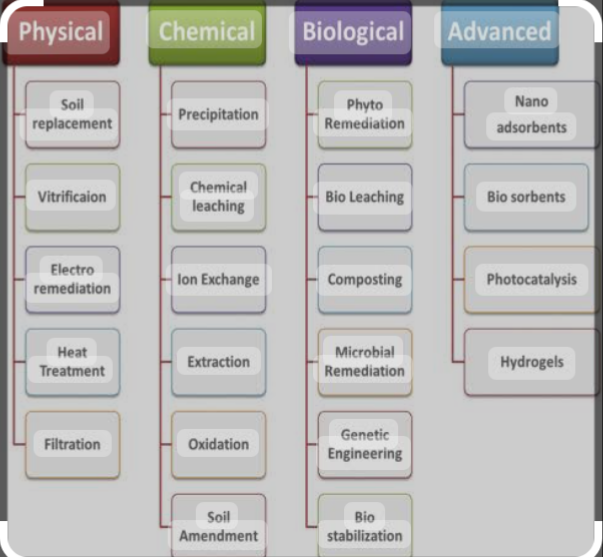
Variety of methods and techniques are used to remove heavy metals from contaminated areas. Such methods include physical, chemical, and biological processes. Physical methods include ultrafiltration, coagulation, flocculation, adsorption, membrane filtration, and ion exchange . Chemical methods include neutralization, solvent extraction, chemical precipitation, and electrochemical treatment. And biological method include bioremediation, biochar and phytoremediation methods.
Total eradication of heavy metals cannot be achieved but reduction to a safe level can be done. During application of treatment methods, subsequent treatment methods should follow initial treatment so as to further reduce the concentration of heavy metals to safe levels. Some of the methods are explained below;
1. MEMBRANE FILTRATION.
Membrane filtration is a process that involves the use of a semipermeable membrane to separate contaminants from water. Membrane filtration methods such as reverse osmosis and nanofiltration have been used for heavy metal removal in many reports.
In this method, contaminated water from industries like mining industry is feed into a ceramic membranes to filter it. A feed pump is then used to generate pressure to make the contaminated water move through the membranes. The permeate move through two membrane structure to filter the liquid.
Firstly, the permeate moves through a silicon carbide membrane layer. And
Secondly, the permeate moves through the membrane substrate structure, which is a layer made of larger silicon carbide gains than the membrane coating layer. This complete the heavy metal filtration process and the permeate will end up in a permeate tank without heavy metals and ready for further usage.
2. ELECTROCHEMICAL TREATMENT.
Electrochemical treatment involves the use of an electrical current to induce chemical reactions, resulting in the removal of heavy metals from wastewater. The electrochemical treatment methods such as electrocoagulation, electrooxidation, and electroflotation have been used for heavy metal removal.
3. NANOTECHNOLOGICAL APPROACHES Nanotechnology is the use of interdisciplinary methods of creating nanoscale materials or devices that include concepts from physics, chemistry, engineering, and biology. In nanotechnology, nanomaterials are developed and manufactured to detect heavy metals and removal. Some of the nanomaterials used to remove heavy metal including graphene and its derivatives, magnetic nanoparticles, metal oxide nanoparticles, and carbon nanotubes, to name a few. Using nanotechnology for heavy metal analysis and removal from food and water resources provides many benefits over traditional methods. These advantages include a broad linear range, low detection and quantification limits, a high sensitivity, and high selectivity..
4. MOLTEN SALT THERMAL TREATMENT
Molten salt thermal treatment is an effective method in dissolving heavy metals in municipal solid waste incineration (MSWI) fly ash. Molten carbonates or chlorides are used to extract heavy metals from fly ash, which reduced the leaching toxicity of the heavy metals in the reacted slags. The heavy metals like Cd, Cu and Pb in fly ash are transferred to molten salt. An example of molten salt is NaCl-CaCl2.
5. BIOREMEDIATION
Bioremediation is an environmentally friendly process that cleans and remove harmful contaminants which pollute soil, water, and air using microbes and plants to keep the environment safe. It can be employed along side other waste management methods such as physical and chemical treatment methods for complete management and treatments of heavy metals. Living organisms, like microbes, microalgae, fungi ( mycoremediation) and bacteria and plants ( phytoremediation) are introduced into the heavy metal contaminants site where they assist in removal and cleansing of these harmful byproducts from the environments.
6. PHYTOREMEDIATION,
Phytoremediation is a bioremediation process which involve the use of various types of plants to remove, clean, and/or destroy contaminants in the soil and groundwater. The plant uses its
roots to uptake the pollutants along with water and minerals and translocate them to the shoots and leaves through the xylem and phloem tissues. These plants and their parts which mop up the contaminants are then harvested and distroyed. Phytoremediation is only applicable if the soil contamination does not exceed 3 feet from the surface and 10 feet from groundwater surface. There are different types of phytoremediation. They include rhizofiltration,
phytoaccumulation, phytodegradation, phytostabilization, phytovolatilization, phytotransformation, and a combination of these methods. Examples of such plants used for heavy metal removal include; alfalfa (Medicago sativa ) and ryegrass (Lolium perenne ), Oryza sativa(rice), Pistia stratiotes (water lettuce), Pisum sativum (pea), Brassica juncea (mustard) , pennywort ( Hydrcotyle umbellata), water velvet ( Azolla pinnata), duckweed ( Lemna minor), Sunflower ( Helianthus annuus)
TOXICOLOGICAL IMPACTS OF HEAVY METALS ON THE ENVIRONMENT AND HARMFUL EFFECTS ON THE ECOSYSTEM .
The benefits of heavy metals are generally outweighed by their hazards. They possess detrimental effects on both the environment and living organisms. Most of the toxic heavy metals including lead, thallium, cadmium, and antimony, are common in industrial operations and are substantial polluters of the environment. Heavy metals produce the following effects:
EFFECT ON SOIL
1. Heavy metals alter the composition of soil
2. It affect the activities of soil microbial organisms. They induce toxic effect on the microbs.
3. It causes high soil toxicity
4. It may persist in soil for a long time
5. It affects soil pH, organic matter, charges on soil colloids, and soil surface area,
6. They reduce organic matter decomposition
7. They alter the morphology, metabolism and growth of soil microorganisms
EFFECT ON PLANTS
1. Heavy metal accumulation above tolerance level in plants lead to plant death.
2. It can affect biological and physiological processes required for plant growth.
3. It can result to reduce yield in plant
4. It causes oxydative stress in plant which result to cytoplasmic enzyme inhibition and cell structure damage
5. It can replace essential nutrients at the cation exchange site of plants
6. Plants exhibit stunted growth, deformation, and reduced physicochemical activities
7. Contamination of heavy metals restrict the growth and survival of plants, leading to nutritional, ecological and evolutionary challenges.
8. Heavy metal toxicity can alter the structure of nucleic acids, disrupt cell membranes, interfere with cellular functions, inhibit enzyme activity, and affect energy production in plants.
EFFECT ON HUMAN
1. Thallium has a more severe effect than other heavy metals, but is less abundant in nature. it causes alopecia in humans.
2. High exposure to antimony and chromium causes carcinogenicity in human
3. Lead poisoning causes intellectual abnormalities in children
4. Mercury toxicity causes Minamata disease. methylmercury poisoning, is the most well-known and infected people show symptoms including sensory disturbance, cerebellar ataxia, hearing and speech issues, and narrowing of the visual field. People are infected when they Consume contaminated fish and shellfish, which accumulate the discharged methylmercury, caused the poisoning
5. Cadmium poisoning causes “itai-itai” disease. “itai–itai” disease is an endemic illness contacted from the consumption of rice contaminated with a high level of cadmium poisoning

6. Heavy metals can also cause toxicity in certain organs of the human body, such as nephrotoxicity, neurotoxicity, hepatotoxicity, skin toxicity, and cardiovascular toxicity, among other things.
7. Heavy metals can cause harm to various organs, including the neurological system, liver, lungs, kidneys, stomach, skin, and reproductive systems, even at low exposure levels.
To avoid toxic effects, it is necessary for people to move away from industrial areas where heavy metal emission is considerable
8. In human, it can cause loose intestines, anxiety, lung disease, fatigue, kidney problems, stomach issues, skin infections, neurological issues and malignant growth
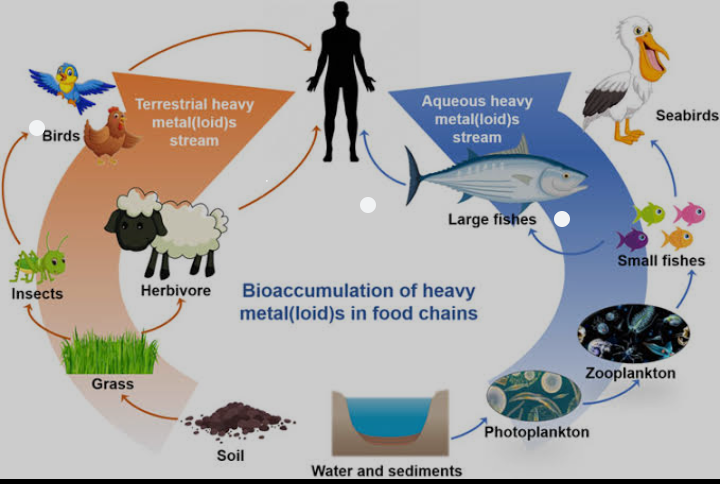
9. The high levels of heavy metals, by contaminating the food chain, can cause severe ecotoxic stress on humans and aquatic organisms.
10. Exposure to heavy metals through food can lead to accumulation in human bones and fatty tissues, leading to nutritional deficiencies and weakened immune responses.
11. Cadmium and lead, among other heavy metals, have been found to be associated with intrauterine growth retardation.