
One of the ways of increasing soil pH is by application of lime. The primary reasons for liming is to neutralise toxic elements in acid soil rather than simply to raise soil pH. The acid cations especially Al and Mn, are phytotoxic at relatively low soil solution concentration. Thus, pH is the primary indicator that predict the toxicity of Al and Mn in soil. Liming through addition of hydroxide (OH-) decreases the solubility of Al3+, Mn2+, Fe3+, Cu2+ and Zn+ causing them to precipitate as sparingly soluble silicates clays, oxides and hydroxide. Excessive application of lime can cause deficiency of these nutrient elements. Heavy application of lime also causes decrease uptake of Boron from soil.
LIME also supplies Ca and Mg depending on the source. Indirect effect of liming include increased availability of P, Mo and B and more favourable conditions for microbial mediated reactions such as nitrogen fixation and nitrification and atimes improve soil structure. Some diseases are decreased while some are enhanced. Liming also improves the effectiveness of several herbicides.
LIMING MATERIALS
Liming materials include calcitic limestone (CaCO3), wood ash, Basic slag (CaSiO3. CaO), Dolomitic lime ( Ca. Mg(CO3) 2), hydrated/slake lime (Ca(OH) 2),Calcium oxide /quick lime / burned lime (CaO), Mail (Amorphous CaCO3), chalk, and miscellaneous materials like ground oyster shell and by product of paper mill. etc.
The selection of any liming material should be based on its ability to neutralise soil acidity, chemical composition, fitness of grind, cost and ease of handling.

HOW LIME NEUTRALISES ACIDIC SOILS
Lime is composed of sparingly soluble calcium and magnesium carbonates. Some liming materials contain calcium oxide and calcium hydroxide which are soluble. When mixed with water, lime dissolves slowly (this depend on the fineness of the liming material) . In the presence of an acid, lime dissolute more rapidly. When calcium and magnesium carbonates are applied to soil, both the calcium and magnesium will help displace the exchangeable acidity from the soil, while the anionic carbonates, oxides and hydroxides components which are the active ingredients in lime materials react with the acidic soil to neutralize it’s acidity. The carbonates and oxides will dissolve in water to form hydroxide.
For calcium carbonate, the reaction is as follows
CaCO3 + H2O——-Ca2+ + HCO3- + OH-
For calcium oxide
CaO + H2O——–Ca+2 + 2OH-
Nutralization of soil acid
H+ + OH- —– H2O
The calcium and magnesium cations in the exchangeable forms are plants nutrients and not involved in neutralizing soil acidity.
EFFECT OF LIME ON CROP PRODUCTION
Crops respond differently to lime application because of differences in their tolerance to soil acidity. Some crops cannot produce under acidic conditions because of the following:
1. Al and Fe toxicity
2. Ca, Mg and Mo deficiency
3. Slow organic matter decomposition
Liming is used to reduce soil acidity. It reduces excess soluble Mg and Fe by causing them to form insoluble hydroxide.
CALCIUM CARBONATE EQUIVALENT (CCE) OF LIMING MATERIALS
CaCO3 equivalent : This is a terminology used to describe the neutralizing power of a liming material in relation to pure CaCO3 which is rated 100. It is an important measurement in determining how much lime should be applied to the soil.
CCE is expressed as a percentage of calcium carbonate, which serves as the standard (100%) and it can be calculated using the formula below;
Molecular weight of CaCO3 * 100
%CCE= ———————————————- ——-
Mole weight of CaO. 1
REACTION OF LIME IN THE SOIL
The following are steps involved when Ca2+ and Mg3+ compound are applied to acid soils, they reaction with CO2 and with acid colloidal complex.
1. REACTION WITH CO2: All liming materials whether oxides, XOH-, XCO- react with CO2 and H2O to yield bicarbonate when applied to an acid soil.
CaO + 2CO2 + H2O——Ca(HCO3) 2
Ca(OH)2 + 2CO2——Ca(HCO3) 2
CaCO3 +CO2. + H2O—–Ca(HCO3)2
2. REACTION WITH SOIL COLLOIDS: liming materials react directly with acid soils. Ca and Mg replaces H+ and Al3+ on the colloidal complex.
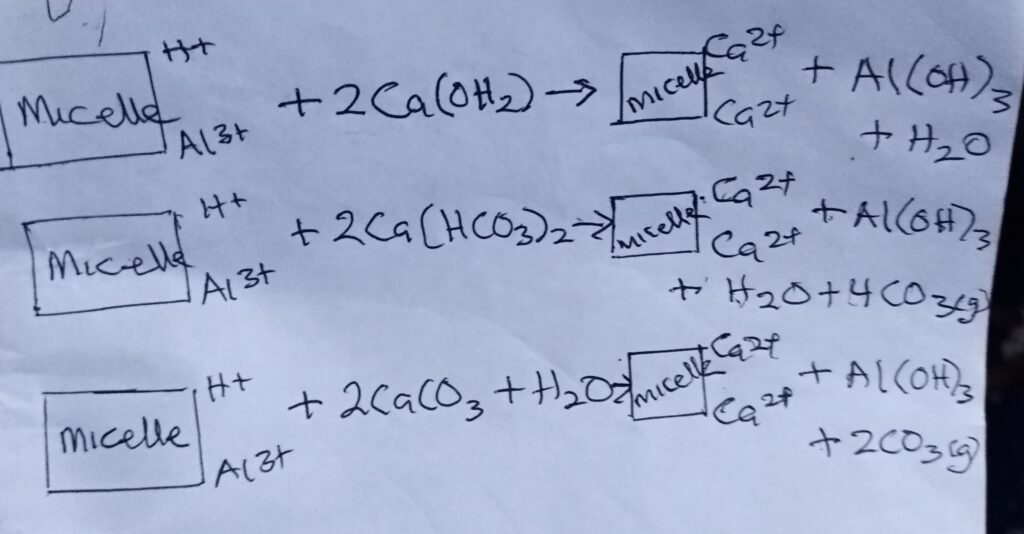
CONDITIONS THAT NECESSITATE THE ADDITION OF LIME OR CAUSES OF SOIL ACIDITY.
1. Annual precipitation exceeding evaporation, resulting in the loss of basic cation (Ca2+, Mg 3+ and K+) by leaching and being replaced by H2
2. Loss of basic cation by erosion (water and wind)
3. Neutralisation of basic cation by continuous use of acid base fertilizers like urea (NH4) 2SO4.
4. Acid rain: by burning high sulphur concentration which produce acid forming smoke.
H20 +SO2——–H2SO4
5. Acid soil develop from acidic parent materials
LIMING REQUIREMENT
This is the amount of liming material required to bring about a desired change in pH. To determine the amount of lime required to neutralise an acidic soil, analysis of soil pH, exchangeable acidity, and knowing the target pH are required. The pH of the soil is a function of the type of exchangeable complex present, and the proportions of CEC which is occupied by acidic and basic cations. As the base cation saturation of soil increases, the pH also increases in like manner. Likewise, as the acid cation saturation increases, the pH of the soil decreases. Liming requirement calculation is based on the relationship between pH and base saturation.

Lime requirements of crops grown on acid soils are determined by the quality of liming material, status of soil fertility, crop species and cultivar within species, crop management practices, and economic considerations. Soil pH, base saturation, and aluminum saturation are important acidity indices that are used to determine liming.
FACTORS THAT DETERMINE LIMING REQUIREMENT
1. THE CHANGE IN pH REQUIRED : the strongest factor that influences yield and pH changes under acidic soil conditions is the liming rate and it was inferred that a higher liming rate will solve issues relating to soil acidity.
2. BUFFER CAPACITY OF THE SOIL : the positive effect of a higher lime rate on soil pH might be due to the high buffering capacity of acidic soils which would thus require a higher lime rate to neutralize acidity.
The quantity of aluminum and hydrogen in acidic soil is not permanently fixed. Instead, the relative amounts of aluminum and hydrogen can change. Thus, the soil is said to have a buffering capacity. Buffering capacity is the ability of the soil to resist change. In the case of acidity, it is the ability of the soil to resist change in pH.
3. CHEMICAL COMPOSITION OF THE LIMING MATERIAL USED : The neutralizing value of any lime material is related to the amount of carbonate or oxide the lime material comprises. A higher neutralizing value indicates higher carbonate or oxide levels in the lime material with better capacity in neutralizing soil pH. The neutralizing value of calcium hydroxide is higher than the other types of lime materials.
4. FINENESS OF THE LIMING MATERIAL :. The degree of fineness of liming material is vital because liming materials with finer particles are more soluble and disperse faster in the soil, while coarse lime materials react slowly.
5. pH AND CATION EXCHANGE CAPACITY (CEC): Soil’s ability to hold and supply nutrients is related to its cation and anion exchange capacities and the number of parking spaces for nutrients on soil particles. Cation and anion exchange capacities are influenced by soil pH and they are largely determined by the charge on the soil particles and soil organic matter (SOM). Soils with high amounts of clay and/or organic matter typically have higher cation exchange capacity (CEC), that is, are able to bind more cations such as calcium or potassium than more silty or sandy soils. They also have greater buffering capacity.
The effect of Soil pH on nutrient availability can be noticed as H+ ions take up space on the negative charges along the soil surface, displacing nutrients.
The metal micro nutrients such as copper (Cu), iron, 4( Fe) , manganese (Mn) and zinc (Zn) dissolved in water with 2 to 3 positive charges. They bind strongly to the surface of soil particles. At high pH (that is, basic or low H+ concentration m), these metal ions stick so tightly in soil solution and thus are less available for plant uptake. At low pH (that is., acidic or high H+ concentration), few of these metals stick to the soil surface because the H+ displace them, making them more available for plant uptake.
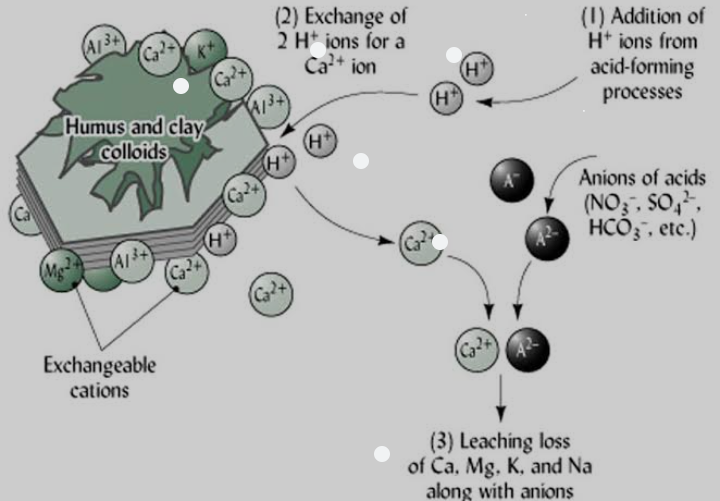
Sulfur (S) and the base-forming cations (Ca2+, Mg2+, K+, and Na+) are relatively macro molecules that do not stick tightly to soil particles. Therefore, even at high pH (low H+ concentration), they easily detach from the soil particle and enter soil solution. At low pH they are displaced by H+, and may not be available to plant because they are leached or uptake by the plants. Nitrate (NO3-) is equally available across soil pH levels because it does not bond much to soil.
In general, nitrogen, potassium, calcium, magnesium and sulfur are more available within soil pH 6.5 to 8, while Boron, copper, iron, manganese, nickel, and zinc are more available within soil pH 5 to 7. At pH less than 5.5, high concentrations of H+, aluminum and manganese in soil solution can reach toxic levels and limit crop production. Phosphorus is most available within soil pH 5.5 to 7.5
6. TILLAGE :
Tillage does not consistently increase or decrease soil pH. The top few inches of no-till soils can become more acidic due to nitrogen fertilization in that zone. Occasional tillage mixes the acidic layer with higher pH sub-surface layers, or helps integrate lime treatment.
6. CROP SELECTION :
Crops vary in their ability to raise or lower soil pH. For example, harvest of high yielding leafy crops such as forage or corn can reduce soil pH because leaves and stems contain large amounts of base-forming cations (Ca2+, K+, Mg2+).
Legumes acidify their rooting zone through nitrogen-fixation. The acidifying potential of annual legumes is lower than that of perennial legumes. Also, plants have ability to uptake nutrients from the soil to develop their tissues. This mining process do result in acidic soil
FACTORS INFLUENCING pH VALUES IN SOIL
1. PARENT MATERIAL : The real pH of newly formed soils is derived from minerals in the soil’s parent material. In environment characterised by heavy rainfall, soil acidification occurs as the products of weathering are leached by water moving laterally or downwards through the soil. While in dry climates, however, soil weathering and leaching are less intense and soil pH is often neutral or alkaline.
2. CLIMATE :
Temperature and rainfall regulate leaching intensity and soil mineral weathering. Soils formed in regions of high rainfall are acidic (low pH value) because the basic cations can be leached down the ground, while those formed in regions of low rainfall are alkaline (high pH value). Rainwater has a slightly acidic pH (usually about 5.7) due to a reaction with CO2 in the atmosphere that produces carbonic acid. When this water flows through the soil, it results in the leaching of basic cations from the soil. This increases the percentage of AI3+ and H+ relative to other cations. In dry climates, soil weathering, and leaching are less severe. That’s why pH can be neutral or alkaline.
3. NITROGEN FERTILIZATION : over time, nitrogen fertilizers can lower pH values. In warm and humid environments, soil pH drops over time in a process called soil acidification.
4. MANAGEMENT FOR PARTICULAR CROP : Soils often become more acidic when crops are harvested and crop residues removed from the field. The removal of the crop residues means removal of basic cations absorbed by the plant.
Type of crop grown also determines the relative amounts of nutrient cation removal. For example, legumes generally uptake higher levels of basic cation than grasses. This gives one of the reasons grasses can survive on any type of soil. When soils are acidic, liming materials’ may be added to raise its pH. But when soil are alkaline, elemental sulfur or acidifying materials may be added to lower the pH for a particular target crop.
5. BUFFERING CAPACITY : This is the soil’s ability to resist change in pH. Soils that have a high clay and organic matter content have high buffering capacity than sandy soils. Organic matter content can be altered by management practices. Sandy soils have a low organic matter content therefore, resulting in low buffering capacity.
6. SOIL MANAGEMENT PRACTICES : The type of vegetation found on a particular soil do have effect on the pH level of that soil. Soils of forest areas tend to be more acidic due to the following factors. Acidification of the soil due to acid deposition, which can lead to decreased soil pH . Another factor is the presence of high nitrogen (N) depositions, which can contribute to soil and surface water acidification . Also, The presence of high concentrations of exchangeable hydrogen (H) ions and aluminum (Al) ions in the forest floor can increase soil acidity. While soils of grassland areas are less acidic. etc.
IMPORTANCE OF LIMING
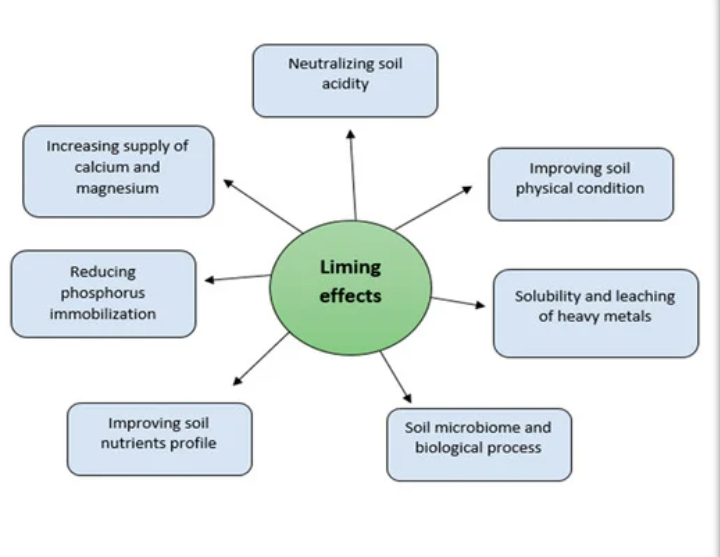
1. Liming improves soil pH, Ca, and Mg contents and reduces Al concentrations in the soil solution.
2. Liming improves beneficial microbe populations in the soil. It enhances microbial activities in soils, triggering the mineralization of soil organic matter and residues
3. Liming improves P concentration in the soil solution by reducing P immobilization by Fe and Al in acid soils.
4. Liming improve the root growth of crop plants. 5. Liming increased shoot dry weight, total root length, and mycorrhizal colonization of roots in soybean and corn grown on tropical acid soils.
6. Apart from changing soil pH, liming can also reduce Cu availability of soil by enabling precipitation of Cu-hydroxides and/or Cu-carbonates.
7. Soil organic matter in limed soils decompose faster and associate with soil minerals than in unlimed soils
8. Lime improve water quality in acidified catchments
9. The dissolution of added CaCO3 improves the bulk water quality in fish farming by increasing the water hardness and pH/alkalinity.
10. Liming acidic soils is generally promoted as an effective management practice to increase soil pH, base cation concentrations, and ameliorate toxicity caused by aluminum and manganese.
11. Liming promotes nitrification and N-mineralization in no-till soils thus increasing soil nitrate.
12. Liming promotes the advancement of earthworm colonization in soils which positively impacts soil structure.
13. Also, previous review studies have shown that lime can be utilized to remediate cadmium (Cd) in Cd-contaminated soils.
14. Lime increase nutrient use efficiency in grasslands within livestock grazing system
15. Liming’ also reduces N2O emissions,
16. Liming increases Ca2+ concentrations and ionic strength in the soil solution, causing clay flocculation and thus an improvement in soil structure and hydraulic conductivity
17. liming increase soil carbon content because it increased crop yields and therefore residue returns.

APPLICATION AND OVER-LIMING
Lime materials can be broadcasted uniformly on the soil surface, after which it should be thoroughly tilled into the soil for optimal neutralization of soil acidity. liming material can only react at the depth to which it was incorporated. To reduce the acidity at other horizons, subsurface tillage may be necessary to get the liming material deeper beyond the top horizon.

Despite the many benefits of lime, over-liming the soil can negatively affect the soil and crop planted on it. It is important to carefully consider how much lime to add to neutralise the acidity. Over-liming can reduce the availability of micronutrient and phosphorus and cause their deficiencies as well as permit molybdenum toxicity. To correct these problems might be difficult. The only alternative method to correct these problems is by reducing the pH through application of sulphur or aluminium sulfate.
To prevent over-liming, soil test should be carried out.

