
The soil pH depends on the rock from which the soil are formed and also the weathering processes involved in soil formation. The weathering processes include climate, vegetation, topography and time. These processes tend to cause a lowering of pH (increase in acidity) over time.
Some agricultural activities can also accelerate the acidification process.
What is pH?
pH is the measure of the active H+ concentration in the soil solution. It indicate the acidity and basicity of a medium. Or Soil pH is the measure of soil acidity or alkalinity, specifically the inverse log of the Hydrogen ion concentration.
equation: pH = -log10 [H+]
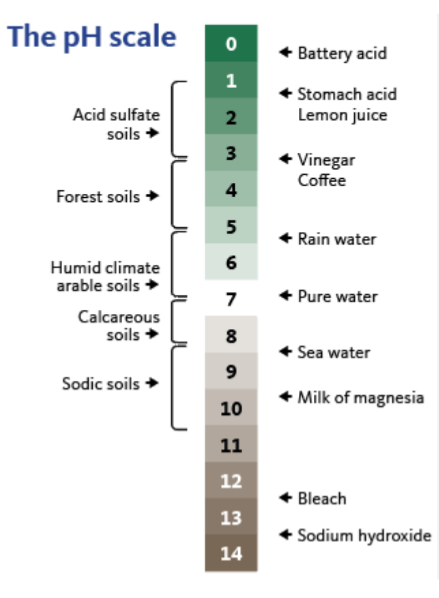
The pH scale ranges from 0 to 14. A neutral state is indicated by pH 7. This pH represent the value found in pure water. Values above 7 are basic while values below 7 are acidic. This means that a very acid soil has a low pH and a high hydrogen ion concentration. And at high (alkaline) pH values, the hydrogen ion concentration is low.
The pH scale is logrithemic, meaning each unit has a 10 fold increase or acidity or basicity. Thus, compared to a pH 7.0, pH 6.0 is 10 times more acidic, and pH 5.0 is 100 times more acidic. These simply means a unit change in pH for example from a pH value of 5.0 to a pH value of 4.0 indicates a 10x increase in H+.
Most soils have pH values between 4 to 10.
The correct balance is where the soil pH is between 5.5 and 7.5, so every effort should be taken to check soil pH levels regularly. Early identification of soil pH problems is important as it can be both costly and difficult to correct long-term nutrient deficiencies.
IMPORTANCE OF SOIL pH
Soil pH has several impacts on plant nutrients and plant growth. Soil pH also impacts or interacts with other properties in the SOIL. Some of the importance of pH include:
1. SOIL pH INFLUENCES AMOUNT AND AVAILABILITY OF PLANT NUTRIENTS :
Plant nutrient availability is stongly tied to the activity of H+ and pH of the soil solution. Some plant nutrients are more available under acidic conditions, while others are more available under basic or alkaline conditions. Decreasing soil pH, directly increases the solubility of Mn, Fe, Cu, and Zn. At pH value less than 5.5, Mn, Zn and Al which are non nutrient elements in the soil becomes toxic. The availability of N, K, Ca, Mg and S tends to decrease with decreasing pH since conditions which acidify the soil such as weathering and plant uptake also result in removal of these nutrients or in decrease microbial activities.
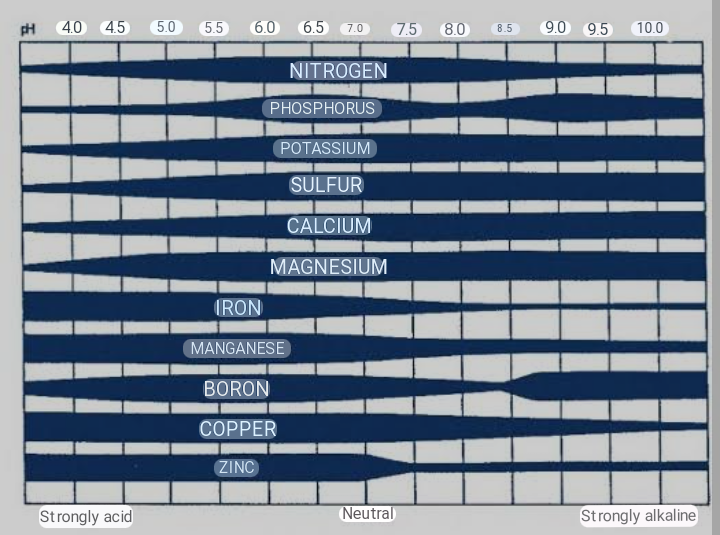
pH also have an indirect effect on phosphorus (P) and Boron (B) . The availability of both nutrients depend on their formation of less soluble compounds with Al, Mn, Ca, and Fe. At very low and high pH, availability of P and B decreases with maximum availability at pH range between 5.5 to 7.0. This makes P to bind more strongly to the soil than B. While B can be leached from the soil.
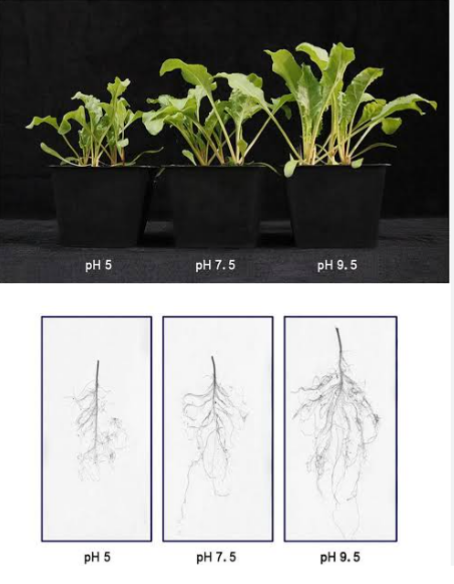
Most mineral nutrients are readily available to plants when soil pH is near neutral. The development of strongly acidic soils (less than 5.5 pH) can result in poor plant growth. This low pH can be as a result of the following factors:
Al and Mg toxicity, Ca and Mg deficiencies, low levels of essential plant nutrients such as P and Mo,. While alkaline soils may have problems with deficiencies of nutrients such as Zn, Cu, B and Mn. Soils with an extremely alkaline pH (greater than 9) are likely to have high levels of sodium.
2. pH AFFECT THE ACTIVITY OF SOIL MICROORGANISMS RESPONSIBLE FOR RESIDUE DECOMPOSITION :
Under acidic situations, the activities of soil microbes are impaired. Most microbial processes, including the breakdown of organic matter and cycling of nutrients, are reduced in acidic soil because growth and reproduction of the soil microbes, primarily bacteria and fungi, are reduced. Micro-organisms break down organic matter and use the carbon and nutrients for their own growth.
The rate of mineralisation of nutrients by soil microbes into plant-available forms is slower in acidic soil, potentially limiting plant uptake.
In addition, legume nodulation can be affected in acidic soil. Legumes which are plants that fix atmospheric nitrogen into the soil through a symbiotic relationship with specialised bacteria will not nodulate in acidic soil. Therefore, fixation of atmospheric nitrogen may not occur. It is only under favourable conditions that nitrogen-fixing rhizobia bacteria form a symbiosis ( partnership) with crop and pasture legumes in root nodules.
Soil microorganism activity is greatest near neutral conditions, but optimal pH ranges vary for each type of microorganism. Microbial activity is considerably reduced at pH 5 and below. Moreover, certain ‘specialized’ microorganisms, such as nitrifying bacteria (convert ammonium [NH4+] to nitrate [NO3-]) and nitrogen-fixing bacteria associated with many legumes, generally perform poorly when soil pH falls below 6. Finally, Fungi do thrive at low pH. Therefore, fungal diseases are more common in acidic soils.
3. pH AND SOIL ORGANIC MATTER : Soil organic matter (SOM) serves multiple functions in the soil, including nutrient retention, water holding capacity, it buffers soil pH change, and soil aggregation and is a key indicator of soil quality. It also influence the soil’s cation exchange capacity.
The decomposition of organic matter can have an impact on soil pH. As organic matter decomposes, it releases anions and cations and organic acids as by-products into the soil. These organic acids can lower the soil’s pH, making it more acidic. The process is referred to as “soil acidification.”
Plant foliage and stems generally contain more anions, so the initial decay causes a soil pH increase. This initial increase in soil pH, especially from high nitrogen plant residue, could be used to reduce H+, aluminum or manganese toxicity in the seedling rooting zone long enough for seedling establishment . Soil microbes further break down the plant material to ammonium (mineralization) which temporarily increases pH. The ammonium gets converted to nitrate (nitrification) which causes pH decrease. If the nitrate is lost to leaching, pH drops even more. In the very long term, microbial decomposition decreases pH.
However, the impact of organic matter decomposition on soil pH depends on several factors like type of organic matter, buffering capacity and microbial activities.
TYPE OF ORGANIC MATTER : Different types of organic matter can have varying effects on soil pH. For example, organic materials like peat and some crop residues are more acidic, while others like wood ash or lime can be alkaline.
SOIL BUFFERING CAPACITY : The ability of the soil to resist changes in pH is known as buffering capacity. Soils with high buffering capacity are less affected by the acidifying effect of organic matter decomposition.
MICROBIAL ACTIVITY : Soil microorganisms can also influence pH by releasing acids during their metabolic processes.
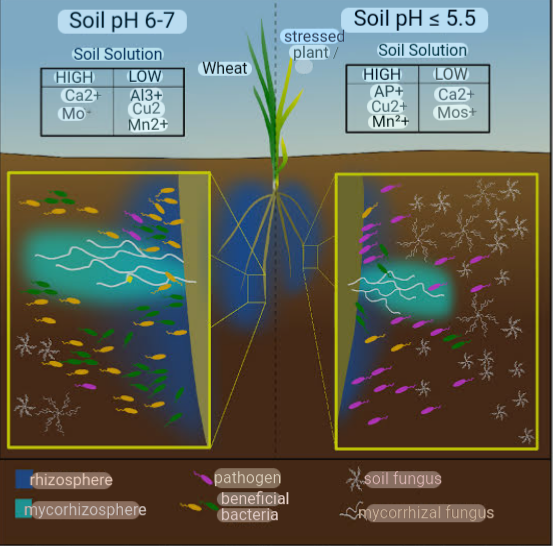
4. pH AND CEC: Soil’s ability to hold and supply nutrients is related to its cation and anion exchange capacities and the number of parking spaces for nutrients on soil particles. Cation and anion exchange capacities are influenced by soil pH and they are largely determined by the charge on the soil particles and SOM. Soils with high amounts of clay and/or organic matter typically have higher cation exchange capacity (CEC), that is, are able to bind more cations such as calcium or potassium than more silty or sandy soils. They also have greater buffering capacity.
The effect of Soil pH on nutrient availability can be noticed as H+ ions take up space on the negative charges along the soil surface, displacing nutrients.
The metal micro nutrients such as copper (Cu), iron ( Fe) , manganese (Mn) and zinc (Zn) dissolved in water with 2 to 3 positive charges. They bind strongly to the surface of soil particles. At high pH (that is, basic or low H+ concentration), these metal ions stick so tightly in soil solution and thus are less available for plant uptake. At low pH (that is., acidic or high H+ concentration), few of these metals stick to the soil surface because the H+ displace them, making them more available for plant uptake.
Sulfur (S) and the base-forming cations (Ca2+, Mg2+, K+, and Na+) are relatively macro molecules that do not stick tightly to soil particles. Therefore, even at high pH (low H+ concentration), they easily detach from the soil particle and enter soil solution. At low pH they are displaced by H+, and may not be available to plant because they are leached or uptake by the plants. Nitrate (NO3-) is equally available across soil pH levels because it does not bond much to soil.
In general, nitrogen, potassium, calcium, magnesium and sulfur are more available within soil pH 6.5 to 8, while Boron, copper, iron, manganese, nickel, and zinc are more available within soil pH 5 to 7. At pH less than 5.5, high concentrations of H+, aluminum and manganese in soil solution can reach toxic levels and limit crop production. Phosphorus is most available within soil pH 5.5 to 7.5
5. TILLAGE :
Tillage does not consistently increase or decrease soil pH. The top few inches of no-till soils can become more acidic due to nitrogen fertilization in that zone. Occasional tillage mixes the acidic layer with higher pH sub-surface layers, or helps integrate lime treatment.
6. CROP SELECTION :
Crops vary in their ability to raise or lower soil pH. For example, harvest of high yielding leafy crops such as forage or corn can reduce soil pH because leaves and stems contain large amounts of base-forming cations (Ca2+, K+, Mg2+).
Legumes acidify their rooting zone through nitrogen-fixation. The acidifying potential of annual legumes is lower than that of perennial legumes. Also, plants have ability to uptake nutrients from the soil to develop their tissues. This mining process do result in acidic soil
FACTORS INFLUENCING pH VALUES IN SOIL
1. PARENT MATERIAL : The real pH of newly formed soils is derived from minerals in the soil’s parent material. In environment characterised by heavy rainfall, soil acidification occurs as the products of weathering are leached by water moving laterally or downwards through the soil. While in dry climates, however, soil weathering and leaching are less intense and soil pH is often neutral or alkaline.
2. CLIMATE :
Temperature and rainfall regulate leaching intensity and soil mineral weathering. Soils formed in regions of high rainfall are acidic (low pH value) because the basic cations can be leached down the ground, while those formed in regions of low rainfall are alkaline (high pH value). Rainwater has a slightly acidic pH (usually about 5.7) due to a reaction with CO2 in the atmosphere that produces carbonic acid. When this water flows through the soil, it results in the leaching of basic cations from the soil. This increases the percentage of AI3+ and H+ relative to other cations. In dry climates, soil weathering, and leaching are less severe. That’s why pH can be neutral or alkaline.
3. NITROGEN FERTILIZATION : over time, can lower pH values. In warm and humid environments, soil pH drops over time in a process called soil acidification.
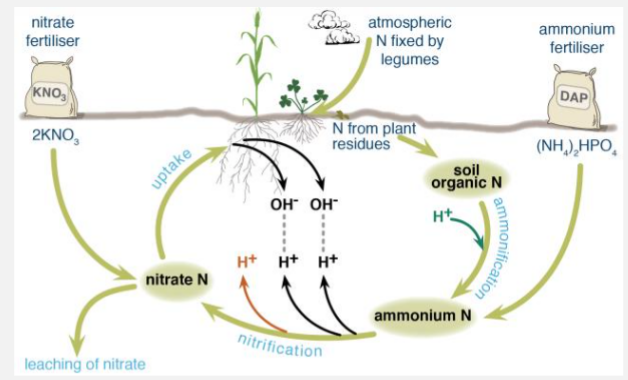
4. MANAGEMENT FOR PARTICULAR CROP : Soils often become more acidic when crops are harvested and crop residues removed from the field. The removal of the crop residues means removal of basic cations absorbed by the plant.q
Type of crop grown also determines the relative amounts of nutrient cation removal. For example, legumes generally uptake higher levels of basic cation than grasses. This gives one of the reasons grasses can survive on any type of soil. When soils are acidic, liming materials’ may be added to raise its pH. But when soil are alkaline, elemental sulfur or acidifying materials may be added to lower the pH for a particular target crop.
5. BUFFERING CAPACITY : This is the soil’s ability to resist change in pH. soils that have a high clay and organic matter content have high buffering capacity than sandy soils. Organic matter content can be altered by management practices. Sandy soils have a low organic matter content therefore, resulting in low buffering capacity.
6. SOIL MANAGEMENT PRACTICES : The type of vegetation found on a particular soil do have effect on the pH level of that soil. Soils of forest areas tend to be more acidic due to the following factors. Acidification of the soil due to acid deposition, which can lead to decreased soil pH . Another factor is the presence of high nitrogen (N) depositions, which can contribute to soil and surface water acidification . Also, The presence of high concentrations of exchangeable hydrogen (H) ions and aluminum (Al) ions in the forest floor can increase soil acidity. While soils of grassland areas are less acidic etc.
SOIL pH MANAGEMENT
To manage soil pH, the addition of amendments like fertilizers and tillage practices, SOM, liming, sulphur and crop selection should all be considered in the management of soil pH
1. APPLY SULFUR AMENDMENTS :
Sulphur is an amendment used to lower the pH of basic soils . It is the cheapest and an organic method that can be used to lower soil pH. Elemental sulfur is oxidized by microbes to produce sulfate (SO42-) and H+, causing a lower pH. The H+ acidifies the soil and the sulfate ion remains as a plant food or else is leached by rainwater or irrigation. Ferrous sulfate (FeSO4) and aluminum sulfate (Al2[SO4]3) can also be used to lower pH. The acidic cations (Fe2+, Al3+) are responsible for lowering the pH, while their sulphate are used as plant nutrients. Both compounds are expensive to purchase compared to use of elemental sulphite. Application rates for these amendments vary depending upon soil properties (particle size, oxidation rate) and soil conditions (original pH, buffering capacity, minerals present). Carbonates such as calcium carbonate (CaCO3) usually buffers soil to pH values near 8. Soils containing carbonate requires larger quantities of sulfur amendments to lower its pH.
2. LIME APPLICATION
As the base cation saturation of soil increases, the pH also increases in like manner. Likewise, as the acid cation saturation increases, the pH of the soil decreases. Soil pH is the measure of the relative intensity or ratio of base cation saturation of the exchange site. This two also relate to lime requirement.

A common method for increasing soil pH is to lime the soils. Some liming materials include calcium carbonate (CaCO3) , calcium oxide (CaO), calcium hydroxide (Ca[OH]2), or calcium containing by-products such as sugar-beet lime. The selection of liming materials should be based on its ability to neutralize soil acidity, chemical composition, fitness of grind and ease of handling. The liming material reacts with carbon dioxide and water in the soil to yield bicarbonate (HCO3-) and hydroxide (OH-), which take H+ and aluminum (acid-forming cations) out of solution, thereby raising the soil pH.
CaCO3 + H2O—–Ca+2 + HCO-3 + OH-
The acid in the solution is neutralized
H+ +OH- —- H2O
3. FERTILIZER APPLICATION
Nitrate-based nitrogen fertilizers, such as calcium nitrate (15.5-0-0 +19% Ca) may increase soil pH at both the surface and deeper levels except if the nitrate is uptake by the plant and is not lost to leaching . In contrast, ammonium-based fertilizers, such as urea (46-0-0) and ammonium phosphates can slowly lower pH of basic soils,
Nitrogen fertilizer do cause soil acidification, hydrogen ion (H+) , aluminum (Al+3)and manganese (Mg+3) toxicity, especially when excess of the fertilizers are added to crops. To minimize soil acidification due to nitrogen fertilizer, practices that prevent excess nitrogen application, encourage uptake of all applied nitrogen, and reduce nitrate leaching should be embacked upon
REASONS FOR MONITORING SOIL pH AND HOW TO ADJUST IT.
Before any attempt to change soil’s pH, the current level in the soil must be known. This will determine if there is need to change the pH or how much material is need to raise or lower it. A simple soil test can be done at home or by a soil-testing laboratory. Also texture of the soil must be known, whether it is clay, sand, silt or loamy etc. More material is needed to change the pH level of a clay soil than for a sandy soil because the charged surfaces of clays make them more resistant to pH changes than the uncharged surfaces of sand particles.
Once the pH level of the soil is adjusted in readiness to plant, such pH should be maintained. Factors that affect pH should be put to consideration. For example, rainfall leaches out calcium and other alkaline-forming elements. Fertilizers can change soil pH over time, especially when materials such as ammonium sulfate and ammonium nitrate are applied. They lower the soil pH level. While potassium nitrate or calcium increases the soil pH. This therefore calls for a need for regular additions of limestone or sulfur.

