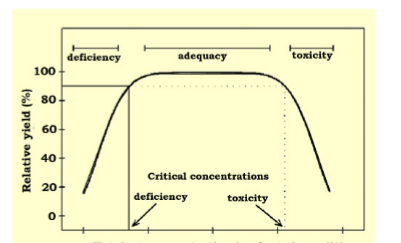
Plant analysis when combined with soil analysis gives an indication of soil health from which plants draw nutrients. These two analysis is used for fertilizer recommendation program. Plant analysis is sometimes required to explain plant nutritional disorders and symptoms observed on the field. It is also required for interpretation of field experiment for adversary proposes and survey of nutrient deficiency. Although soil test has being more widely used than plant tissue test to diagnose nutrient deficiency. Plant analysis therefore has some unique advantages.
ADVANTAGES OF PLANT ANALYSIS
1. Nutrient concentration in plant tissues is a reflection of available nutrients in the soil.
2. It is a better measure of soil – plant interaction than soil test
3. Micronutrients deficiencies are better diagnosed in plant tissue analysis than by soil test
Plant tissue analysis differ from tissue testing. Tissue testing typically refer to a field test that involves collecting sap samples from fresh plant tissues and analysing that sample on site. Plant tissue analysis is performed on dried leaves tissues processed in the laboratory.
TYPES OF PLANT ANALYSIS
There are two types of plant analysis. They include:
1. Total or quantitative analysis
2. Semi quantitative or relative quantitative analysis
1. QUANTITATIVE ANALYSIS: This types of analysis measures both the elements that have being incorporated into the plant tissue and those that are still present in the soluble constituent of the plant sap
2. SEMI QUANTITATIVE ANALYSIS: It is also called rapid tissue test. It measures the unassimilated soluble content of the plant sap. This is the constituent that are measured enroot from the point of entry to the site of utilization within the plant.
USES OF PLANT ANALYSIS
Plant analysis is used for several purposes.
1. It is used to diagnose or confirm diagnoses of visible symptoms. Tentative identification of plant nutrient deficiency symptoms becomes more confidently confirmed after plant analysis or tissue test
2. To identify hidden troubles: Many nutrient deficiencies produce no specific symptoms on plants order than a general loss of vigor or reduced yield. Plant tissue analysis serve as a trouble shooting tool to diagnose a suspected nutrient deficiency. Some types of plant analysis can be used to identify this hidden troubles. Steps are there after taken to correct them.
3. To locate areas of incipient or beginning deficiencies: Visible deficiency symptoms are rearily useful guide to locate fertility experiment especially with micronutrients until the deficiencies are relatively acute. Either the total of the relative quantitative test of the plant sap may be capable of indicating shortages of nutrients that are not severe enough to produce recognizable symptom.
4. It is also used to indicate whether the applied nutrient entered the plant. When nutrients are applied to correct deficiencies, it is helpful to know whether the nutrients are actually micro nutrients or macronutrients and whether the nutrients are taken up by the plant. Otherwise when no yield response is obtained, it may be concluded that the nutrients are not lacking whereas they may not have being taken up.
5. To indicate interactions or antagonism among nutrients. Sometimes the addition of certain nutrients reduce the amount of other nutrients in the plants. Additional data from plant or hybrid of thesame crop analysis will accelerate the development of solution to such practical problems in the application of and other nutrients.
6. It is to understanding internal functioning of the plant. Periodic analysis of whole plant or plant parts through the season under varied environmental conditions show greater differences among crops and even among varieties or hybrid of thesame crop. Plant analysis are useful in showing the mobility of elements within the plants and the concentration which may suggest where the greater needs for certain nutrients exist for metabolic processes.
7. It is also needed to suggest additional test inorder to identify the trouble. For example, sometimes plant analysis point to the need of soil test to identify the specific cause of trouble like in a plant analysis where the content of manganese was usually high, soil analysis shows that the pH was low and therefore, the soil can not satisfy the growth of maize. This is because low pH contribute to low manganese content of the maize plant
PROBLEMS ENCOUNTERED IN THE USE OF PLANT ANALYSIS
1. The technique for sampling, washing, and preserving the samples are not standardised. There is variability among apparently healthy plant in a field and this need to be adequately understood so as to serve as an adequate guide to the number of plants to sample for different levels of precision in test result. Washing of plant samples is usually a problem particularly when problems are diagnosed on field where suitable detergents and distilled water are not available. Soil particles, chemical sprays and dust must be rapidly removed as they lead to misleading or unusual result. In the cause of removal, certain soluble nutrients may be washed out of the tissue. Preservation of plants tissues may depend on the particular analysis to be carried out since some chemical changes begins at once after the plant or plant parts are severed or cut off from the remainder of the plant or root system. For all analysis, it is desirable to minimize cell respiration, mould growth and bacterial decomposition. This process reduce the dry weight of the plant or tissue and this change the percentage of the elements that are to be measured
2. SUITABLE STANDARD : There is need to improve on the reference standard taken into consideration, the correct plant part to be sampled and also the age of the plant.
3. Analysis sometimes fail to indicate the cause of the trouble. Sometimes plant analysis may not show the cause of deficienencies probably the core samples, when not taken at the right time. In such situations, soil test will be short coming indicating the problem
3. PLANT ANALYSIS : Are usually post mortem. Plant analysis can rearily be made early enough in the season to serve as a guide for correcting the conditions in the correct growing season with fertilizer treatment. However, the result may be very useful in planning treatments in or for future treatments.
4. Interpretation is often difficult because the plant analysis measures only the inorganic constituents, at one point in time where as the composition, vigor and health of the plant are the cumulative result of the plant total environment whether soil aeration and compaction, pH and the relative supply of other nutrients influence plant composition more frequently. Only incomplete information is available to the person who is expected to interprete the test result.
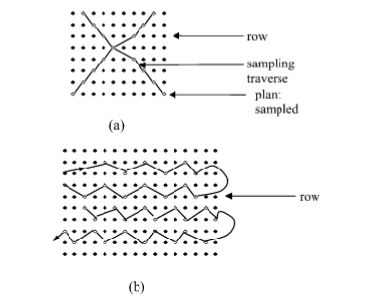
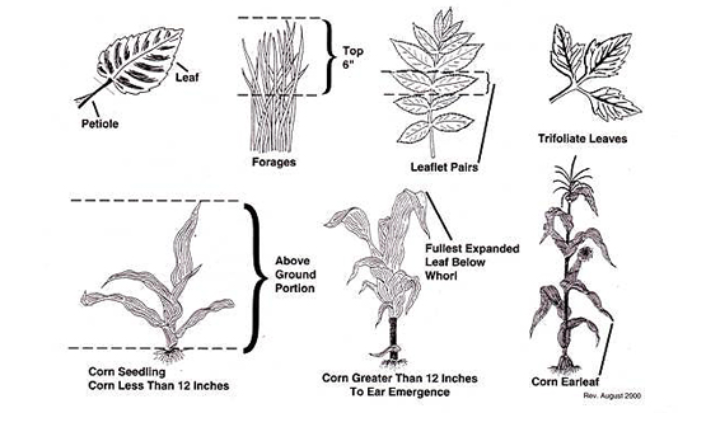
PLANT SAMPLING: Procedure for sampling field crops can be determined from one crop to another. Field and vegetable crops are differently sampled From plantation crops. In general, leaves are preferably sampled to other parts of the plant. Mature leaves immediately below the growing tips of main stems or branches should be sampled. The best time to sample is when the plant is about moving from vegetative growth phase to reproductive growth phase. Leaves covered with soil particles, dust, damaged by insects, injured or diseased should not be sampled. Dead leaves or tissues should be discarded.
FACTORS CONSIDERED WHEN SAMPLING
1. Sampling is not recommended when plants are under moisture or temperature stress.
2. Seed is not a valuable part of the plant to diagnose it’s nutrient status.
3. Do not sample when pod setting or fruits and seed development has commenced. At this stage, there are substantial changes in the nutrient concentration of the vegetative parts. This makes tissue analysis result difficult to interprete. 4. Plant samples should not be bulked or composited. Samples of plants from one area should be kept separately from other areas.
5. Also, one should attempt to sample atleast10% of the plant in a given area but note, sample a minimum of 5 plants per plots.
HOW CROPS ARE SAMPLED
1. SELECTION OF PLANTS AND PLANT PARTS: Plant nutrient levels with the growth stage and the different parts of the plant are selected for sampling . Therefore, when taking plant samples for analysis for fertilizer recommendation program, the plant growth stage and at sampling is important. Also, the nutrient levels can vary from one part of the plant to the other. Plant nutrient sufficiency levels have been calibrated to certain growth stages and parts of the plant.
Generally, plant samples should be taken to the laboratory immediately to minimize deterioration. If this is not visible due to long distance between field and the laboratory, the sample should be placed in porous containers. The commonly used is big paper bag or paper envilope to allow air circulation thereby keeping the temperature at a minimum level and reducing the rate of respiration, thus, minimizing the break down of organic constituents.
2. WASHING: It is almost impossible to obtain dust free plant samples from the field. Therefore, it may be necessary to rinse the sample. Washing of plant sample will depend on the extent of contamination by dust particles. If the contamination is much, it may be adviseable to wipe the samples with a damp cloth especially if accurate data is required on iron and manganese from the samples. Under field conditions, contamination found on plant materials will not normally alter the concentration of most nutrients significantly.
3. DRYING: This is aim at minimizing chemical and biological changes if drying is unduly delayed. It may lead to considerable loss in dry weight due to respiration and protein are broken down to simpler nitrogeneous compounds. Drying at too high temperature can also affect the dry weight. Drying therefore must satisfy two important requirements:
a. A sufficiently high temperature must be employed to destroy the enzymes responsible for decomposition process
b. The optimum temperature for moisture removal without appreciable thermal decomposition must be employed.

Therefore, it is suggested that samples should be dried at between 60 to 80 oC. The enzymes are considered inactive only if the materials are heated to above 60oC. Therefore, air dried plant materials is not recommended. It is advisable to dry in a dust free, forced draft electric oven. Greater thermal decomposition occur when leaf powder are dried at higher temperature than when plant samples are in its original physical states at thesame temperature.
Calculation of arsh content:
Ash content (%) = (x3-x1) / (x2-x1) *100
Crusible is the container where plant samples are put
Crusible =x1g
Weight of Crusible + plant material in furnace =x2g
Weight of crucible + Ash = x3
4. GRINDING: Mechanical grinding is done to reduce the material to a fine state suitable for analysis. This is also done partly for greater ease in manipulation and partly to ensure greater uniformity in composition because of the laborious nature of hand grinding, particularly where samples are large, mechanical grinding is more favourable. However, it is of utmost importance to consider the possibilities of contamination when selecting the mail. Care must be taken when grinding samples. Fineness is of considerable importance as this seems to ensure that the samples are homogeneous in particle size. It is also important to mix thoroughly inorder to control static electricity during grinding.

5 STORAGE: In storing the plant materials, it is important to make adequate provision for keeping and maintaining the quality of the plant materials when dealing with large numbers of plant samples. There may be a considerable delay before the plant samples can be washed and dried. Fresh plant materials does not decompose significantly for a day or two if air dried prior to laboratory handling. However, such materials are difficult to clean properly. If stored under refrigeration, they can be cleaned easily and efficiently. When transporting by mail, they should not be sealed in packages but opened to the atmosphere. Transport should be made as quickly as possible. Dried and grinded samples should not be stored on the shelf longer than two months before analysis. The materials can however be stored indefinitely in a sterile steeped bottle refrigerated at 0 to – 5oC in the dark.
WASHING OF PLANT TISSUES: With the possible exception of sodium, Phosphorus , Chlorine and nitrates, all of the essential elements are integral parts of the plant tissues. They are not present as water soluble inorganic cation or anion. Therefore the organic matter of the plant tissues has to be destroyed before these elements can be determined. This extraction can be accomplished by means of arshing.
There are two types of arshing
1. Dry arshing
2 wet arshing
WET ARSHING: This involves the decomposition of the plant material in a mixture of strong acids consisting of nitric acid, sulphuric acid and perchloric acid (HClO4). There is need to take caution steps inorder to prevent explosion when the perchloric acid is used. Wet digestion( arshing) is preferably for the analysis of trace elements and the elements that are likely to be lost by volatilization during dry washing. For example, nitrogen, chlorine, and sulphur. Wet digestion is not applicable to boron analysis because it can be lost by boric acid. Similarly, boron can be lost by volatilization as phosphoric acid when digested with perchloric acid and if the temperature exceed 230oC.
DRY ARSHING: This is the combustion of plant sample at a sufficient high temperature to burn off organic matter. However, if the heating is too rapid and the temperature is too high, the sample may burst into flame. Therefore, temperature should not exceed 500oC and it should be done for 2 to 8 hours. Too high temperature should be avoided for the following reasons
1. Sodium, potassium, chlorine, sulphur and boron are lost by volatilization as organic compounds. For temperature below 600oC, no loss of phosphorus is expected while at temperature up to 800oC, no loss may be expected if calcium or magnesium acitate or nitrate is added to the samples before sampling.
2. Addition of lime or sodium carbonate to the sample before arshing prevent the loss of chlorine and sulphur. Loss of boron can be prevented by the addition of NaOH to the sample
3. Fission of salt in the marsh can occur and complex silicates which are not readily soluble in HCl acid can also form, as some plants containing appreciable amount of silicon. Such silicate complexes if formed, might involve substantial amount of trace elements and possibly some sodium and potassium. However, silica can be dissolved by hydrochloric acids to release the occluded elements
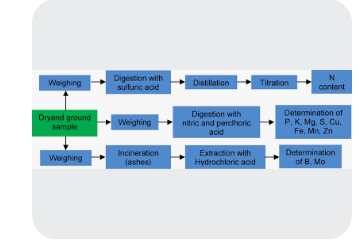
ANALYTICAL PROCEDURE
Sodium and potassium in the digest can be determined using flame photometry. Calcium magnesium, iron, manganese, zinc and cupper can be determined using atomic absorption spectrophotometry (AAS). Phosphorus by Colorimetry ( spectrometer) and sulphur by turbidometry. Nitrogen by kjeidahl procedure. Tissue analysis of any element in the plant is total of quantitative analysis. It enumerate the total content of that particular nutrient in the plant at that specific stage of growth. The relative or semi quantitative analysis is therefore different and it is used to study specific processes in the plants. For example, in the study of nitrogen fixation, methods used include, nitrogen difference method, ethylene acetate method, isotropic method.
However, the method of choice recently developed is the Ureide method
UREIDE METHOD OF NITROGEN DETERMINATION
The xylem sap living the root of a full grown legumes carry nitrogen compound to the shoot.
SOURCE OF NITROGEN ORIGINATING FROM THE XYLEM SAP
1. Nodules as assimilation product of nitrogen fixation
2. Soil mineral nitrogen taken up by the root.
In some legumes such as soybeans, cowpea, and mung bean, the xylem solutes exported from the nodules containing ureids and Allantor are very different from the compounds transported from roots (Amino acids and nitrate). In this species, the abundance of ureids relatively to the other nitrogen compounds in the xylem samples is indicative of plant dependence upon nitrogen fixation. Ureid production appear to be restricted to certain taxonomic groups of legumes including cowpea, soybeans, chicken pea, and lablab etc. The ureid method involves the recovery of the xylem sample by applying a mild vaccum and measuring the three nitrogen components of the xylem sap separately by simple colorimetric assays and then to determine the plant reliance upon nitrogen fixation for growth. This is calculated by relating the determined xylem sap composition to glass house prepare calibration curve.