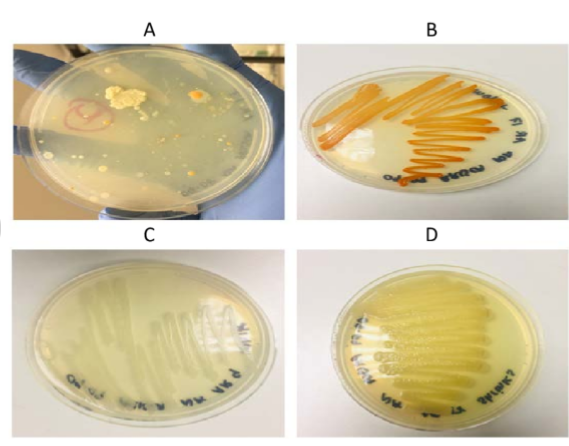
CULTURAL TECHNIQUES FOR SOIL MICROORGANISMS
Soil organisms are responsible for performing vital functions in the soil. They make up the biodiversity of life In the soil. Soil biodiversity which is an important component of the ecosystem comprises of organisms that spend all or part of their life cycle within the soil or on the soil immediate surface such as surface litters and decaying logs. These organisms carry out a range of processes involve in improving soil health and fertility. Some of them feed on living plants and animal ( herbivores and predator), some on dead plants debris ( detritivores), some on fungi and bacteria and some are parasitic in nature.
Soil microorganisms are the smallest organisms with size of <0.1mm in diameter. They are extremely abundant and diverse. They include algae, bacteria, cyanobacteria, fungi, yeast, myxomycetes and Actinomycetes. The assist in the decomposition of any existing natural materials. They transform organic matter into plant nutrients that are assimilated by plants.
IMPORTANCE OF SOIL MICROORGANISMS
1. Bacteria contribute to carbon cycle by fixation (photosynthesis) and decomposition
2. A free living bacteria called rhyzobium assist in fixing atmospheric nitrogen into the soil
3. Actinomycetes do form an association with non legumineous plants and fix nitrogen
4. Bacteria produce exudes which is a sticky substance in the form of polyssacharide that helps bind soil particles into small aggregates. Thus conferring structural stability to the soil
5. Bacterias also help improve water infiltration and water holding capacity of the soil
6. Saprophytic Fungi convert dead organic materials into fungal biomass, carbon dioxide and other small molecules like organic acids
7. Mycchorizal fungi forms mutualistic association around plant roots for plant to reach nutrients and water that otherwise might not be available
8. Many fungi helps control diseases
9. Some fungi are biocontrol agents that feed on insects.
10. Phytophages or plant feeding nematodes damage plant roots, thus, causing economic consequences for farmers.
Soil micro organisms may be beneficial and at thesame time harmful. Therefore, studing microorganisms like bacteria or fungi by scientists is important. They are grown or cultured in an ‘artificial’ laboratory setting. This gives better understand of the organisms and their possible applications to nature, as well as what they are sensitive to and how to destroy them if need be.
IMPORTANCE OF CULTURING SOIL MICROORGANISMS
1. Cultivating and studying microorganisms gives better understanding of them ( morphological, physiologically and their activities)
2. In medicine, Microorganisms that cause infection in sick patient need to be dentified so as to give proper treatment. By isolating and culturing this microorganism from patient samples, solutions are provided to ill health in the patient. Bacteria culture tests are often used for this purpose to help find harmful bacteria in the body and diagnose bacterial infections. In fact, bacteria are the most common microorganism cultured in a laboratory . Patient samples taken include: blood, urine, stool, mucus, skin or even spinal fluid for microorganism culturing and investigation. This allows doctors to identify the microorganisms and decide the best way to treat the infection.
3. When culturing bacteria we can test their antibiotic susceptibility and treat infections based on this information
4. Bacterial culture is used to measure the effectiveness of antibiotics, antiseptics or disinfectants by calculating the size of the zone of inhibition around the substance being tested. The zone of inhibition is the area that is not colonised by bacteria due to the presence of antibiotics.
5. Culturing microorganisms is also important for research purposes that involve genetic manipulation of the microorganism, for epidemiological studies or to develop vaccines and other therapeutics.
6. Culturing microorganisms is essential to detect any possible food contaminants. Harmful bacteria like Salmonella and Campylobacter can contaminate food and be responsible for food poisoning.
7. Culturing microorganisms is important applications in biotechnology. Microbial biotechnology plays an important role in the health, agriculture, chemical, energy, environment and food and beverage industries.
8. Soil microorganisms are handle and cultured safely in a laboratory because they can multiply quickly and some of them can be very harmful to human.
CULTURE OF MICROORGANISMS IN LABORATORIES
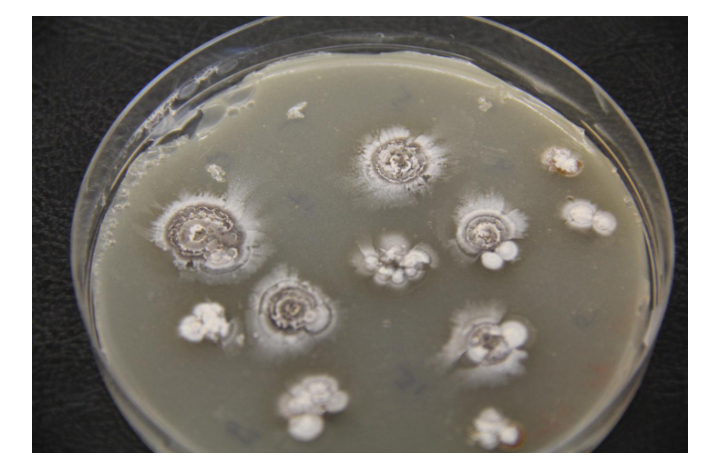
Culturing involves multiplying microorganisms in a controlled way and under laboratory conditions. In order for culturing to work, scientists must be able to mimic the environmental conditions needed for these microorganisms, so that they not only survive but also grow in a laboratory setting. Everything the microorganism needs must be provided and controlled to optimized standards. Conditions that must be controlled for successful microbial cultures include: Temperature, pH, Sterility and Nutrients
FACTORS REQUIRED FOR SUCCESSFUL CULTURE OF MICROBS
1. NUTRIENTS: Nutrients can be provided to the microorganism by using a culture medium. This medium can be in either liquid or solid (gel) form. It provides the surface for the microorganisms to grow on and contains all the essential nutrients. These usually include minerals, a nitrogen source (for protein production) and carbohydrates (energy source) and other chemicals that the microorganisms would usually find in nature.
2. AMPLE TEMPERATURE :
Microorganisms also require warmth and oxygen to be able to grow, but not all have the same temperature requirements and some may even be able to grow in the absence of oxygen
3. SUITABLE pH: pH is also important as it affects the structure of macromolecules so it must be kept to the microorganisms’ preferable range.
4. SUBSTRATE: This is a growth medium for micro organism and from which they get their nutrients. For example, agar

5. STERILITY: sterility refers to the absence of contaminating viable microorganisms.
CULTURE METHODS AND TECHNIQUES
Cultural technique are those methods involved in the transfer of propagules of the microorganisms to a substrate conducive to growth. All cultural techniques are selective and designed to detect microorganisms with particular growth form or by chemical capabilities. It also provide a general estimate of the numbers and types of organisms in the soil. Their biomass and the function they perform.
SAFETY PRECAUTIONS IN CULTURING SOIL MICROBS
1. STERILE TECHNIQUES IN CULTURING SOIL MICROBS
Sterile techniques is the name given to important safety procedure which must be followed wherever cell cultures are handled in the laboratory.
AIMS AND OBJECTIVES OF STERILE TECHNIQUES
i. To prevent accidental contamination of laboratory culture due to microbs from external sources. For example, skin, clothing or the surrounding encironment
ii. To prevent microbial contamination of laboratory workers.
2. HANDLING OF CELL CULTURE
There are four major reasons why a special care is required in handling cell culture
i. . A harmful microbs may be accidentally isolated as a contaminant when culturing a relatively harmless strain
ii. Some individuals are more susceptible to infection and diseases than others
iii. Laboratory culture involves unified and growing large numbers of microbial cells. This represent a greater risk than small numbers of the original microbs
iv. A microbs may change it’s characteristics perhaps as a result of gene exchange or mutation
STERILISATION PROCEDURE
Given the inhibuity of microbs, the only way to achieve a sterile stage is by extraction or removal. Several methods can be used to achieve this objectives and this include the following:
1. HEAT TREATMENT: This is the most widely spread form of sterilization employed in several basic laboratory procedures and this could be done in different ways.
a. RED HEAT STERILIZATION: This is achieved by heating metal innoculating loops, forecepts, and needles etc in a burnsen flame. This is a simple and effective form of sterilization as no microbs will survive even in brief exposure to naked flame. Flame sterilization using alcohol is used for glass rods and spreaders.
b. DRY HEAT STERILIZATION : Here hot air is used at a temperature of atleast 160oC for atleast 2 hours. This method is used for routine sterilization of laboratory glass wears. Dry heat procedure are of little value for items requiring repeated sterilization during use.
c. MOIST HEAT STERILIZATION : This is the method of choice for many laboratory items including most fluid apart from heat sensitive media. It is also used to decontaminate liquid media and glass wears after use. The laboratory autoclave is used for this purpose
Typically, most items will be sterile after 15 minutes at 121oC, although, large items may require a longer period.
The rapid killing action result from the latent heat of condensation of the pressurized steam released on contact with cool materials in the autoclave.
2. RADIATION: Many disposable plastic items used in microbiology and cell biology are sterilised by exposure to ultraviolet or ionising radiation. They are supplied commercially in sterile packages ready for use. Ultraviolet radiation has limited use in the laboratory, while ionising radiation like gamma rays requires industrial facilities and cannot be operated on a laboratory scale
3. FILTRATION: Heat labile solution ( for example, complex macro molecules including proteins, antibiotics cerum) are particularly suited to this form of sterilization. The filters come in a variety of shapes, sizes, and materials. Usually with a pore size of either 0.2 micrometers or 0.45 micrometers. The filtration apparatus and associated equipment is usually exhausted and sterile by autoclave or by dry heat. Passage of liquid through a sterile filter of pore size 0.2cm into a sterile vessel is usually sufficient to to remove bacteria but not viruses. So filtered liquids are not necessarily virus fluid.
4. CHEMICAL AGENTS: These are known as disinfectant or biosite and are most often used for disposal of contaminated items following laboratory uses. For example, glass slides and pipette. They are also used to treat spillages. The term disinfection implies extraction of diseases caused by bacterial cell. Although, spores and viruses may not always be disturbed. Remember that disinfectant require time to extract their killing effect. Any spillage should be covered with disinfectant and left for about 10 minutes before mopping up.
USES OF LABORATORY EQUIPMENTS
1. WORKING AREA: One of the importance of good sterile technique is to keep the working area as clean as possible. Starting by clearing all items from the working surface, while then disinfect using disinfectant. Then the items should be arranged for a particular procedure so that they are close to hand, living a clear working space at the centre of the bench.
2. MEDIA: Cells may be cultured in either a liquid medium like broth or a solidified medium like agar. The gelly agent used in most solidified media is agar. A complex polysaccharide from red algae that produces a stiff transparent gell when used at 1 to 2% weight by volume.
Agar is used because of its rheological properties ( an agar medium melt at 98oC). Once melted, it does not solidifies until the temperature falls to about 44oC.
3. INOCULATION LOOP: The initial isolation and subsequent transfer of microbs between containers can be achieved using a sterile inoculation loop. Most teaching laboratories uses nichrome wire loops with metal handle. A wire loop can be sterilized by heating the wire, looped downward and almost vertical in the hottest part of the burnsen flame until the whole wire becomes red hot. The loop is removed from the flame to minimize heat transfer to the handle. After cooling for 8 to 10 seconds ( without touching any other object), it is ready for use. When resterilizing a contaminated wire loop in a burnsen flame after use, it is better to soak the loop for a few minutes in disinfectant.
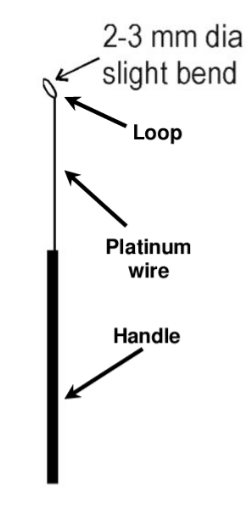
4. CONTAINERS: One method of reducing the chance of air borne contamination ( whenever a sterile bottle, flask or test tube is open) is to pass the open mouth of a glass vessel through a flame. This distroys any microbs on the outer surface nearest to the mouth of the vessel. It is a general practice to flame the mouth of each vessels immediately after opening and then repeat the procedure just before replacing the top. Caps, lids and cotton wool clogs must not be placed on the bench during flaming and sample (they should be removed and held using the smallest finger of one hand to minimize the risk of contamination
5. LAMINAR FLOW CABINET: These are designed to prevent air born contamination. For example, when preparing media or subculturing microbs, sterile air is produced by passage through a high efficiency particulate air filter ( that is, HEPA filter). This is then directed over the working area either horizontally towards the operator or downwards. The operator handles specimen media etc through an opening at the front of the cabinet.

GENERAL RULES IN THE LABORATORY
1. Take care with sharp instrument including needles and glass pasteur pipette
2. Do not pour waste culture down the sink, they must be autoclaved
3. Put other contaminated items ( slide, pipette) into disinfectant after use
4. Wipe down your bench with disinfectant after work is completed
5. Always wash your hand before living the laboratory
PROCEDURES IN CULTURING SOIL MICRO ORGANISMS
1. SAMPLE COLLECTION AND PREPARATION: A representative sampling that have being treated statistically must be obtained. In a fairly uniform area, minimum of four replicates is required in reducing error to about 10% of the measurement. Most samples can be stored at either the field temperature or 4oC.
2. ISOLATION OF MICRO ORGANISMS: Before microorganisms are transfered to propagules, they need to be isolated first from their natural habitat. There are various ways to isolate microorganism. Such ways include:
a. MICROSCOPIC ISOLATION : It involves the picking out of single cell spores or hyphal fragment on a microscopic stage equipped with micro manipulator.
b. ISOLATION FROM PLANT ROOT. FOR EXAMPLE RHIZOBIA : Some plants are used to trap specific microorganisms. For example Rhizobia is isolated from the nodules formed on the root of a susceptible legumes plant like soybeans, cowpea and pigeon pea etc.
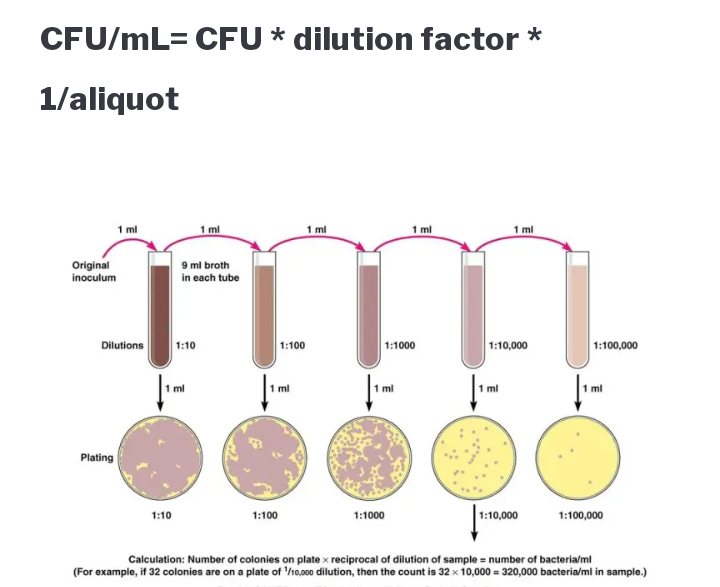
c. DIRECT ISOLATION FROM SOIL : This is done through the use of suspension with the degree of dilution required and related to the initial number of organism in the soil. For example, serial dilution
3. PLATING TECHNIQUE: Many cultured medium make use of solidified medium within a petri dish. A variety of techniques can be used to transfer and distribute the organism prior to incubation. The three most important procedures include:
a. POUR PLATE METHOD : This procedure uses cells in suspension but require moulting agar medium to prepare a single petric plate ( that is, 15 to 20ml) maintained in a water bath at 45 to 50oC.
b. SPREAD PLATE METHOD: This method is used with cells in suspension either in a liquid growth medium or in an appropriate sterile diluent. It is one method used in quantifying the number of viable cells or colony forming units (CFU) in a sample after appropriate diluent
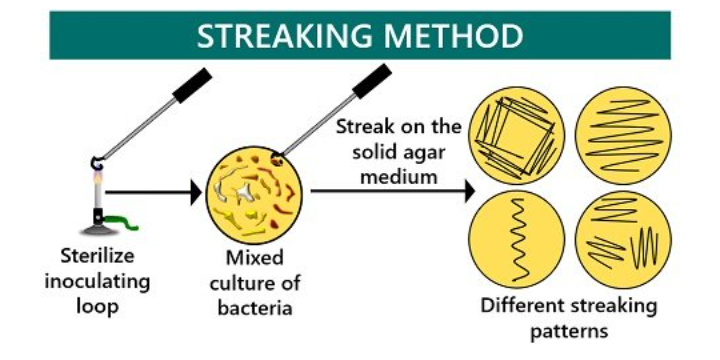
c. STREAK DILUTION PLATE METHOD: Streaking of the plate for dilution colonies is one of the most important basic skill in microbiology since it is used in the method
Stulk culture where a streak dilution plate with single colonies, all at thesame time confirms the purity of the stream
4. INCUBATION: After plating, the plates are stored in the incubator at varying temperature depending on the type of micro organisms being cultured
5. PLATE COUNT: After the determination of the dilution, which exhibit between 30 to 300 colonies, plate count is done using the colony counter, hand lens or dissection microscope. The most widely used method of measuring growth are based on cell numbers
a. DIRECT MICROSCOPIC COUNT : One of the simplest method is to count the cell in a known volume of medium using a microscope or counting chamber or imocytometer. While this gives a rapid assessment of the total cell number. It does not discriminate between living and dead cells. It is also time consuming as a large number of soil must be used
b. ELECTRONIC PARTICLE COUNTER: This instrument gives a direct total count of a suspension of the microbial cell. The Coulter counter detect particle due to changes in electrical resistance when they pass through a small glass tube. It gives a rapid count based on larger numbers of cells than direct microscopic method. Its limitation is that it lacks dissemination between living and dead cells, cell lumps and inanimate particles (dust).
CULTURE BASED COUNTING METHOD: A variety of culture based technique can be used to determine a number of microbs in a sample. The most widely spread approach is to transfer a suitable amount of the sample to an agar medium, incubate under appropriate condition and then count in the resulting colonies. The most accurate result may be obtained for plates containing 30 to 300 colonies. Then determine the mean colony count per plate at this particular dilution “C”
To calculate the colony count per ml of the particular dilution, divide the volume in ml of liquid transfer to each plate “V”
Calculate the count per ml of the original sample by multiplying the reciprocal of the dilution ( that is, multiplication factor “M”. For example, for a dilution of 10-3, the multiplication factor will be 10^3. For soils, food or other solid samples, the gram should be expressed as per gram of the sample. The complete equation for calculating the viable count is
Count per ml (or per gram) = (C/V) * M
For example, for a sample with a mean colony count of 5.5 colonies per plate for a volume of 0.05mls at a dilution of 10-7
Solution
C=5.5, V= 0.05 and dilution =10-7
Cont per ml =(5. 5/0.05) * 10-7=1.1 *10^9 CFUml-1
Strictly speaking, the count should be reported in colony forming unit per ml other than a cell per ml. Particularly in filaments microbs or organisms with tendency to aggregate.
CFC is a cell or group of cells giving rise to a single colony on a solidified medium..