
Phosphorus is an essential macronutrient, though it is required in low quantity. It is critically required at early stage of development of plant and in energy transfer within the plant (glycolysis) through out the growing season. Phosphorus stimulates young root development and early fruiting. It is also essential in several biochemicals that control respiration, photosynthesis, cell division, processes involved in plant growth and development and affect seed formation. Phosphorus is highly concentrated in seeds and fruits. It’s deficiencies are more noticed during vegetative growth and yield than in quality in the plant.
Phosphorus (P) do become fixed to the soil, making it unavailable for plant uptake. The stronger the bonding of phosphate ions to soil components, the lower the availability of P to plants.
The level of moisture in the soil at the time of application of P has a significant effect on how much P can be available to the plant. After uptake of the available P, the residual phosphorus remains in the rooting zone and will be slowly available to succeeding crops.
SOURCES OF PHOSPHORUS
Phosphorus always occurs as the phosphate ion. The principal combined forms in nature are the phosphate salts. The primary source of P in soil is appetite. It is a calcium phosphate that contain hydroxide or fluoride. This mineral is found in bones and teeth. Other sources include decay plants and animal residues, microorganisms and humus. Fertilizer sources include: superphosphates, mono ammonium phosphates, Diammonium phosphates, Ammonium polyphosphate, phosphoric acids, superphosphoric acids and bone meal etc.
Phosphorus can be divided into two groups. There is the water soluble P that dissolves only in water. Citrate soluble P, this is the additional P extracted by the neutral, normal citrate. The sum of water soluble P and citrate soluble P is called available P. The P remaining after extraction with water and citrate is called citrate insoluble P. It may not be readily available to the first crop but it may become available to later crops.
FORMS IN WHICH PHOSPHORUS IS UPTAKE
Plants uptake phosphorus as HPO4- – – and H2PO4- forms. These two forms are limited only to solutions. They both depend on soil pH. In acidic soils (pH 4.3 to 7.0), H2PO4- forms is common. While in alkaline soils (pH above 7), HPO4- – – predominate. High concentration of phosphorus can be seen in youngest plants which have active growing tissues. As the crop matures, phosphorus tends to move and becomes concentrated in the fruits and seeds.
PHOSPHORUS DEFICIENCY SYMPTOMS
Plants deficient in P becomes stunted, and show abnormal dark green colouration. At severe state, the foliage, stem and stalk will show reddish-purple colouration.

Phosphorus translocate from older tissues to newly developed ones. Therefore, discoloration appear on older tissues first before the young ones. Visual detection of phosphorus deficiency symptoms may prove difficult. Only soil and plant analysis can be used to determine the deficiency.
PHOSPHORUS IN SOIL
Among all anions, phosphorus is the only anion that has low mobility and available in soil. It is difficult to manage because it react so strongly with both solutions and solid phase of the soil. This makes mobility in all soil to be extremely limited except in organic soils or white bleached sand with extremely low CEC.
In acidic soils, iron and aluminum solution, oxides and hydroxide forms react strongly with added P, binding it so that it becomes unavailable to plants. The P can be made available by adding organic matter to the soil, this reduces P fixation reaction. The organic matter will make Al, Ca, and Fe bind and form soluble complexes with the P. In alkaline soils, P react with Calcium to form sparingly soluble calcium phosphate. When P fertilizer is added to the soil, not all will be uptake by the first crop, some will remain as residue in the soil which will be available to the later crop planted on the soil.
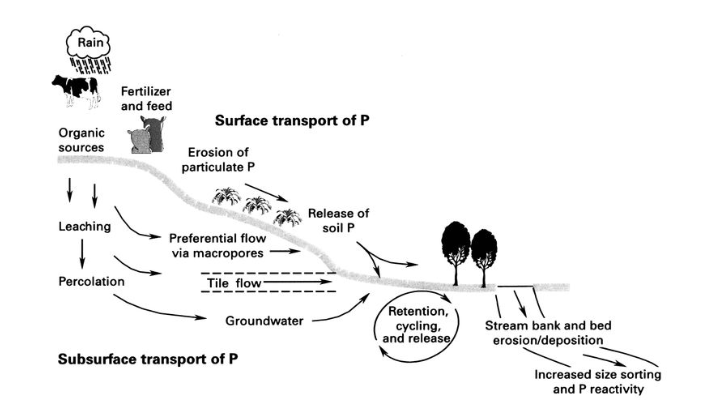
Because P becomes fixed to the soil, they are not easily leached, but become pollutant to surface waters. The P is washed into the water through erosion. As the P gets in contact with water, it’s availability increases.
Intensive fertilization of cash crops, application of manures and biosolids application to soil can make P available to crops even if some are fixed for years.
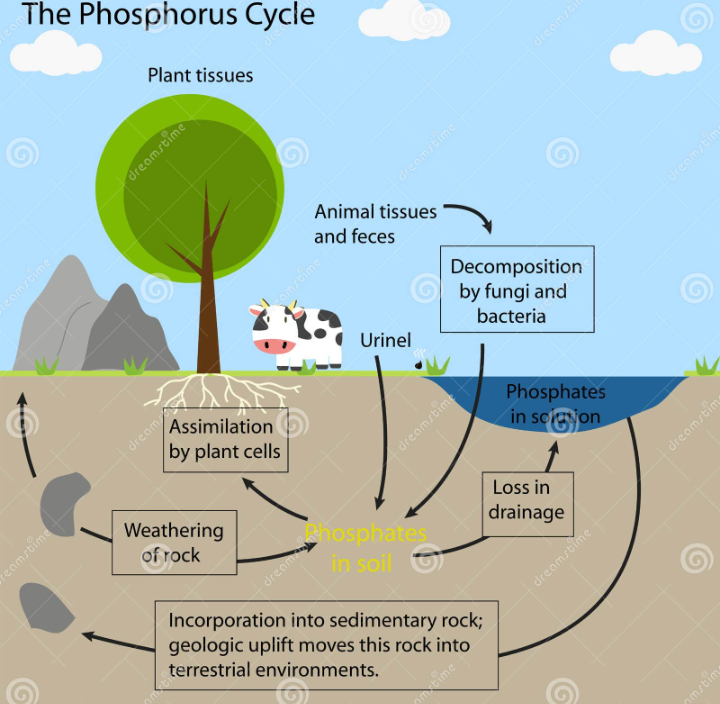
PHOSPHORUS CYCLE
Phosphorus movement and losses can be controlled in the soil through the knowledge of phosphorus cycle. This cycle is a chemical and microbiological reactions that control phosphorus availability in agricultural soils. Phosphorus in soil originates from weathering of minerals in rocks, phosphorus fertilizers, manures, plant residues and agricultural wastes or biosolids. Phosphorus bearing minerals are pH dependent. Phosphorus react with iron (Fe ) and aluminum (Al) to form insoluble Fe and Al phosphates in acid soils and calcium (Ca) to form insoluble Ca phosphate in alkaline soils.
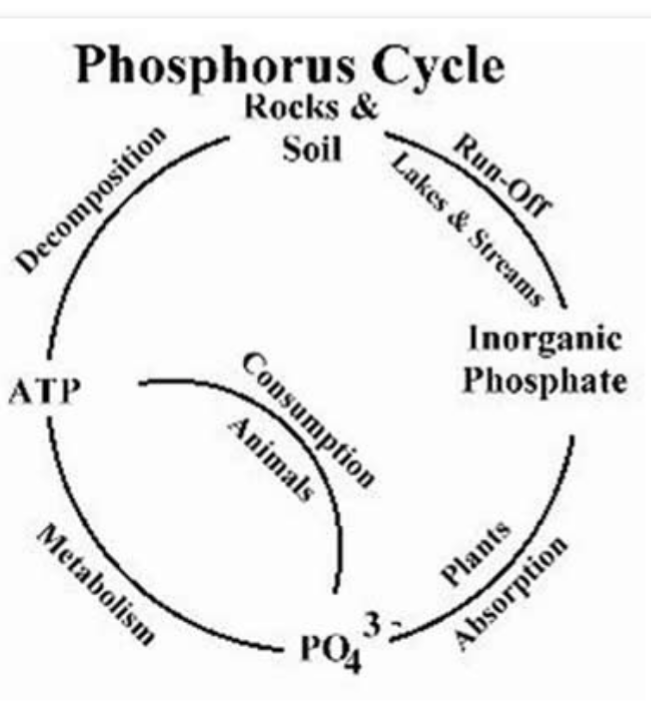
Several chemical and biological processes control the release of soil phosphorus to plant roots for uptake and it’s movement to surface water. When phosphorus bearing minerals dissolve, inorganic phosphate from the mineral is distributed in soils and water. Plants then take up the inorganic phosphate from the soil. Animals consume the plants and the phosphate is released into their body system . Once in the plant or animal, the phosphate is incorporated into organic molecules such as DNA. When the plant or animal dies, organic matter are formed. The organic matter mineralizes ( bacteria break down organic matter to inorganic forms of phosphorus). The phosphorus bound to soil colloids and with time desorb while other phosphorus become released into soil solution. When phosphorus fertilizers is applied to soils, the Phosphorus quickly bound to soil colloids and become fixed. Thus, making the Phosphorus unavailable to plants. Only low amount can be made available to plant.
FACTORS AFFECTING AVAILABILITY OF PHOSPHORUS
Phosphorus availability depend on factors related to interaction with soil components. And also ability of plant roots to take up P from the soil.
1. AMOUNT OF CLAY : Increased clay content result in greater retention of P by the soil
2. TYPE OF CLAY : Kaiolinites and iron oxide retain more P than 2:1 clays. Clays of the 1:1-type (kaolinite) have a greater phosphorus-fixing capacity than the 2:1-type clays (montmorillonite, illite, vermiculite).
3. SOIL pH : This determines the form of P, and level of Al, Ca, and Fe in soil solution. Maximum availability of phosphorus generally occurs in a pH range of 6.0 to 7.0.
4. SOIL PHOSPHORUS LEVEL : Soils very high in P will saturated binding sites and tend to increase high concentrations of P to soil solutions.
5. TEMPERATURE AND RAINFALL : At low and high temperature, ability of the plant to remove P is decreased. Soils formed under high rainfall and high temperatures contain large amounts of kaolinitic clays, and therefore have a much greater fixing capacity for phosphorus. These high climatic factors also increase the amount of iron and aluminum oxides in the soil, which contributes greatly to the fixation of phosphorus added to the soil.
6. MOISTURE : Low moisture do limit the movement of P and affect crop development. Excessive soil moisture reduces the soil oxygen supply which affect plant rooting and limit P uptake.
7. SOLUBILITY AND /OR PARTICLE SIZE : Retention tends to increase as the solubility increases and as particle size of the applied P decreases
8. AERATION : Poorly aerated and poorly drained soils can limit root system development. Poor soil aeration also decreased phosphorus absorption by the plant.
9. TIME OF APPLICATION : Increasing the time of contact between phosphorus and soil particles, increases the amount of P retained in the soil in unavailable form. The efficient utilization of fertilizer phosphorus is generally obtained by applying the fertilizer shortly before planting the crop. This practice is effective on soils with high phosphorus-fixing capacities.
10. METHOD OF APPLICATION : Banded application may be used for applying P fertilizers to soils with high P retention to make the P available to crops when the soil P level is low. Broadcasting application are cheaper, more rapid method, can provide larger rate of P without causing crop injury and can result in better mixing at the root zones etc.
EFFECT OF PHOSPHORUS ON THE ENVIRONMENT
1. EUTROPHICATION : Phosphorus can be washed into water bodies increasing the fertility of the natural water. This can accelerate the growth of algae and other aquatic plants. Even low level of P can cause excessive increase in fertility and productivity of natural waters.
2. Excessive P can cause accelerated growth of algae and water plants which can cause surface scums of algae, foul odour, insect problems, impede water flows and boating due to excessive weed growth.
3. Excessive P causes vegetation and Algal bloom in water to decay rapidly, thus reducing oxygen in water(anoxia) and death of aquatic fishes
4. In agriculture, release of P is greatly reduced as soil erosion is reduced
5. Management practices such as tillage, terracing, grassed waterways, buffer strips, incorporation of manures and fertilizers and crop removals etc all reduce the release of P.
USES OF PHOSPHORUS
1. Phosphorus is applied in the manufacture of fireworks, and matches.
2. It is used as an alloying agent
3. It is used to kill rodents
4. It can be converted to sulfides used in making matches and in the manufacture of insecticides and oil additives.

5. It can be converted to halides or oxides for synthesizing organic phosphorus compounds.
6. The largest use of phosphorus compounds is for fertilisers. Ammonium phosphate is made from phosphate ores. The ores are first converted into phosphoric acids before being made into ammonium phosphate.
7. Phosphorus is also used in the production of steel.
8. Phosphates are ingredients in some detergents, but are being restricted in some countries. This is because they can lead to high phosphate levels in natural water supplies causing unwanted algae to grow.
9. Phosphates are also used in the production of special glasses and fine chinaware.
10. Phosphorus is essential to all living things. It forms the sugar-phosphate backbone of DNA and RNA. It is important for energy transfer in cells as part of ATP (adenosine triphosphate), and is found in many other biologically important molecules.
11. Phosphorus is required for bone and teeth formation since our bones and teeth are mainly calcium phosphate.
12. Over-use of phosphates from fertilisers and detergents can cause them to pollute rivers and lakes causing algae to grow rapidly. The algae block out light penetration, stopping photosynthesis. Oxygen dissolved in the water will get used up and the lake dies.
FACTORS THAT RESULT TO LOSSES OF PHOSPHORUS
1. CROP UPTAKE : Crops uptake phosphorus as one of the essential nutrient required for growth and development. As this phosphorus is uptake, the level of phosphorus in the soil is reduced. 2. CROP REMOVAL : Also, when crops uptake phosphorus from soil solution, the Phosphorus are stored in their tissues. When the plant parts such as fruits, leaves etc are harvested, leaving no decaying vegetation parts, phosphorus is removed from the soil and field.
3. RUNOFF AND EROSION : Water movement across the soil surface can wash away both soluble (that is, dissolved) and particulate forms of phosphorus elsewhere like surface waters to cause pollution.
4. LEACHING : Phosphorus are easily fixed to the soil making it unavailable to plants. This phosphorus can dissolve in water and become leached down the soil horizon. When water table rises, and the soil is saturated with phosphorus, leaching can occur.
CONTROL OF PHOSPHORUS LOSSES
Some farming practices can be emback upon to prevent and control phosphorus losses. Such practices include:
1. CONTROL EROSION : Erosion can be controlled by planting cover crops, conservation practices like conservation tillage, strip cropping, mulching etc.
2. FERTILIZER APPLICATION METHODS : Phosphorus fertilizers can be injected, incorporated or mixed with soil thoroughly to make it bound to soil and reduce its solubility.
3. Phosphorus fertilizers should be applied directly to actively growing crops for the crop to uptake it before becoming fixed
4. NUTRIENT MANAGEMENT PLANS : Farmers should emback on nutrient management plans that will prevent excessive application of phosphorus fertilizers to the soil
5. SOIL TEST : Before any planting operations, farmers should carry out a soil test to determine the level of nutrients in the soil, what nutrient is required and at what quantity so as not to over apply a particular nutrients that result in excess in the soil.

Pingback: buy high pagerank links
Hi,
Thank you for reading through posts on supremelights website. We are grateful. Also, thank you for introducing the pageant link product to us. We will check it out and see how it can value our posts.
If you have any aspect of agriculture you want supremelights to address, please contact us.
Thank you once again.
Bye
If you