
Rocks are composed of minerals, which during weathering parent material are formed. Further break down of parent material form soil. During the weathering process, particle sizes are reduced and many primary minerals are dissolved. Those dissolved minerals are reformed to give a more stable secondary minerals. Minerals such as feldspars, micas and honblend are broken down to form clay minerals. Micas is reduced to clay size particles of less than 2 micrometers. The extreme small particles are called colloid which is the seat of chemical reactions in the soil
WHAT IS A COLLOID ?
Soil colloids are the finer size fractions of the soil
which are small size soil particles less than 0.001 mm or less than 1micrometer in diameter which include clay and humus (organic matter) . Clay is the smallest minerals particles in the soil. When soil has a lot of clay or humus, the soil is said to have high colloidal fractions. All chemical and biological reactions happen within the colloidal fractions. Colloids have a large surface area to volume ratio. This makes them to be extremely reactive. ( that is, the reactive fraction of the soil). It has high surface area with high reactive surfaces. The External surface area is high ( 10m2/g) and the Internal surface area is extremely high( 800m2/g).
Colloid can be classified as mineral or organic Colloids. In all soils, mineral Colloids exceed organic Colloids in quantity. But in organic soils, organic colloids exceed mineral Colloids. Both of the two classes of Colloids account for all the charges and chemical reactivity of the soil. This affect nutrients availability in soils.
Mineral surfaces carry electrostatic charges. This can make the minerals bond to other charges or other materials in the soil. The clay in the soil has a negative electrostatic charges on their surfaces. But in strong acidic soils, positive charges dominate the soil. Water can bond to ions in the soil. And water itself carries a positive charge

TYPES OF SOIL COLLOIDS
There are two different types of soil colloids, they include the organic Colloids ( humus) and the inorganic or mineral colloids(clay)
1. INORGANIC OR MINERAL COLLOIDS
There are two types of inorganic Colloids. They include the crystalline and amorphous Colloids.
a) CRYSTALLINE INORGANIC COLLOIDS
Inorganic Colloids (clay) are crystalline. The crystalline inorganic Colloids can be divided into two: crystalline silicates and crystalline Nonsilicates and
b) AMORPHOUS (NO CRYSTALLINE ).
The amorphous is also divided into two: amorphous silicates and amorphous non silicates.
Inorganic colloids are further divided into three groups.
1. Phyllosilicates or layer silicates clay. They include kaolinites, illite, montmorillonite, vermiculite and smectite
2. Oxides clay. They include iron and aluminium oxides such as Geothite, Hematite and Gibbsite
3. Amorphous clay. Include Allophane and imogolite
2. ORGANIC COLLOIDS OR HUMUS.
Organic Colloid is formed from decomposed organic materials. It is made up of humic and fluvic acids. It is the most complex organic molecule found in the soil and this makes it the most stable organic molecule. It has high CEC range than clay.
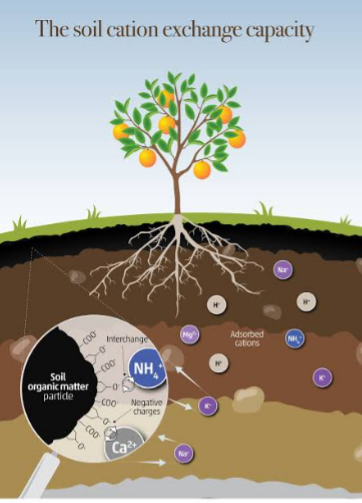
Soil colloids has a large surface area with presence of electrical charges (+or-) on its surface. The electrical charges of the soil affect the attraction or repulsion of particles towards or away from them. This affect the physical and chemical properties of the soil. Most of soil colloids are negatively charged. The colloid has two surfaces, the internal surface which are negatively charged and the external surface which attract positively charged cations.
When Colloids are formed during weathering, they sink down to other horizons in the soil, especially the subsoil horizon where they work to make nutrients available to deep rooted crops.
Nutrients in the soil carry charges.
The positive charge ions are called CATIONS . It include NH4+, Ca2+, Mg2+, H+, K+, Na+, Al3+, Fe2+ or Fe3+, Mn2+, Cu2+, Zn2+. And the negative charge ions are called ANIONS . They include NO3-, PO4-, SO4-.
Soils with large quantities of negative charge are more fertile because they retain more cations. Whereas, soils with low negative charge can be used to grow pastures.
Cations can be classified into two: acidic (acid- forming) and basic. The common acidic cations are hydrogen and aluminum; common basic ones are calcium, magnesium, potassium and sodium.
CATION EXCHANGE CAPACITY
Cation exchange capacity is the total amount of exchangeable cations available for absorbtion by the plant roots. It can also be described as the amount of available sites which the cations in the soil or soil solutions can bind with the anion or negatively charge Colloids. It is the ability of the soil to hold cations. It is expressed in milligram equivalent per kg of soil (meq/kg). It is associated with the physical and chemical process of nutrients movement within the soil, providing roots with nutrition required for growth. It has to do with the Colloids at the root zones. CEC involves soil colloids, soil solution or water and the root system and it is controlled by soil formation stage, pH range and availability of organic materials. It also act as a buffer for the pH in the soil.
pH is the negative logrithem. pH in the soil simply means the amount of negatively charged sites occupied by H+.
Base saturation. These are negatively charged sites occupied by cation like Na, K, Ca and Mg.
Cation exchange capacity is used to determine or measure how much nutrients the soil can hold . The nutrients are represented by base saturation and they include potassium, calcium, magnesium, hydrogen and sodium. These five elements makes up the cation based in the soil. CEC of 10 is considered common, such soil is preferred for plant growth. lower CEC are found in sandy soils. Such soils with a low CEC are more likely to develop deficiencies in K, Mg, and other cations, while higher CEC tends to be more clayey or loamy soils. The higher the CEC the more nutrients and cation the soil can hold. The composition of the five elements combinations makes up 100% of the base saturation.

Ca is about 65. 75%, Mg 15%, K 3-5%, Na 0.5% and H2 10%
What this means is that 90% of the soil should contain Ca, K, Mg and Na. While the remain 10% is H2. When any of these nutrients is lacking in the soil, 90% nutrients can no longer be got in the soil. Therefore, Hydrogen will take the place of the missing element, thereby increasing hydrogen % from 10% to a higher percentage . This hydrogen increment comes from water added to the soil and air. Therefore, when pH of the soil is mentioned, it is the % hydrogen that is being referred to. The higher the %Hydrogen in the ground, the lower the pH and the more acidic the soil would be. This means that the K, Ca and Mg in the soil is low.
For example, assuming Ca is replaced by H2 in the soil, the level of soil Ca is low while H2 increases and the soil is more acidic. When Ca is added to such soil, H2 is displaced and the soil tends to neutrality. Total displacement of H2 by Ca, makes the soil to be more alkaline. This gives the reason why pH is used to determine the health of the soil. And this gives reason why base saturation is used to determine the hydrogen level of the soil.
HOW CATION EXCHANGE CAPACITY WORKS IN SOIL
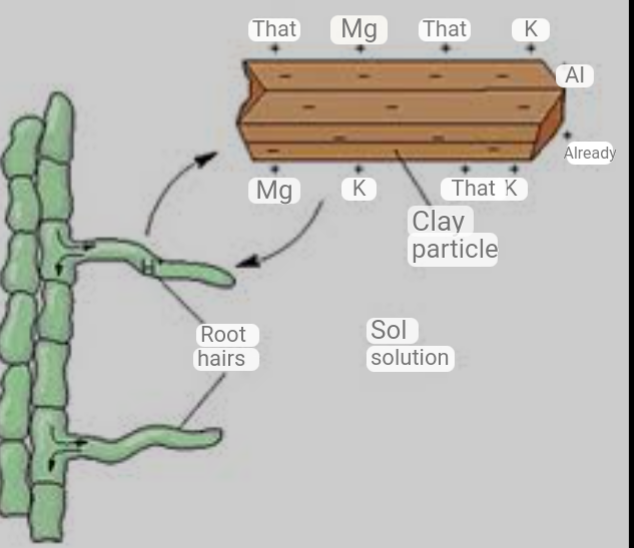
Cation Exchange Capacity works like a magnet. All the soil colloids around the roots are negatively charged. That is, the clay, silt and organic matter are negatively charged. Sand has no charge. While all the cations that will be fed to the plants as nutrients are positively charged. The nutrients added to the soil which are positively charged adhere to the negative charged Colloids. The greater the positively charge, the greater the adhesion of the cations to the soil colloids around the roots. When phosphate fertilizers are added to the soil, the phosphate which is negatively charged adhere to the positive charge cations which bonds to the Colloids. Because the negative charge is greater, phosphorus becomes difficult to remove from the soil. Thus, reason why it get fixed to the soil.
When microbs feed on root exhudats, hundreds of millions of hydrogen ion (H+) are released in large quantities as a by products. The hydrogen ion (H+) readily breaks the bonds that the cations already have within the soil colloids.
Note: The hydrogen ion are acidic cations and therefore easily break the bond that holds the alkaline anion in the rhizosphere. The nutrients are then released for the root hairs to absorb.
CATION EXCHANGE CAPACITY AND SOIL PROFILE

In soil profile, there are different horizons such as O and A horizons which gives the organic layer and top soil respectively, B and C horizons which are the subsoil and parent material, and R horizon which is the bed rock. Some deep rooted crops extend their roots beyond the A horizon to the B horizon to gather and absorb nutrients. Through nutrient leaching and water percolation down the horizon and water translocation, the B horizon gathers clay particles or Colloids, dissolved minerals, organic materials and soil solutions. Note that bacterial are not at the B horizons but at the O and A horizons. They decompose the organic residues to produce nutrients which are leached down to the B horizon where they accumulate.
USES OF CATION EXCHANGE CAPACITY
1. CEC is used as an indicator for soil nutrient level, soil fertility level, level of leaching and pH range.
2. It is used in some engineering fields to measure the capacity of soil to uptake heavy metal cations.
Note: Increase in precipitation result to increase in soil solution and also cause a increase in nutrient flow and uptake through the root system.
IMPLICATION OF CEC ON SOIL MANAGEMENT
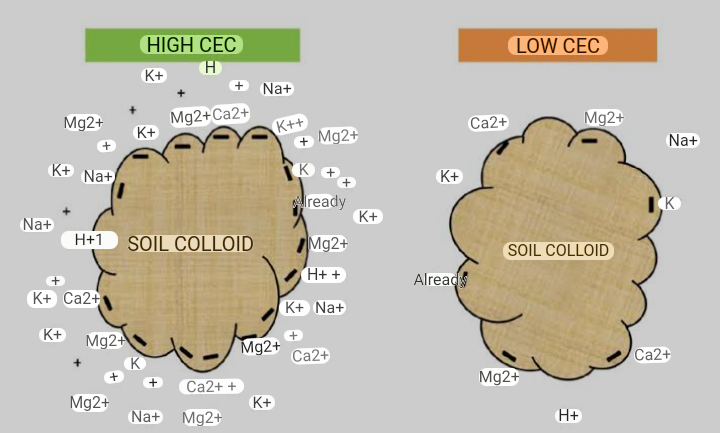
CEC do affect the management of soil for crop production and environmental protection. Soils with low CEC ( less than 5meq/100g) have low clay and organic matter content. The water holding capacity of such soil is low, high leaching rate, and it requires more fertilizers and liming. The advantage of such soil is that they are easy to cultivate, drain more rapidly and any nutrient applied are readily available to the plants.
b) Soils with high CEC (greater than 20meq/100g), have high clay content, high water holding capacity, requires less fertilizers and limes, and leaching of cations is low. The soils are prone to potassium fixation.
HOW TO CALCULATE CEC
Milli equivalent of extract able acidity are determined using a buffer procedure. While the milli equivalent of K, Ca and Mg are determined by extraction.
The equivalent weight of a cation =its atomic weight in grams divided by its valence.
A milli equivalent =1/1000th of an equivalent. (it is expressed in milligrams)
Example 1: The meq of Ca would be=
40g/mole *1000mg/g= 20mg/me
————————————
2eq/mole *1000meq/eq
40g/mole is the atomic weight
2eq/mole is the charge on Ca2+
20mg/me is the milli equivalent weight
Example 2: if a soil is found to contain 80mg of Mg in 100g of soil. The meq of Mg present would be?.
Solution:
80mg of Mg/100g of soil =6.67meq of Mg/100g – – – – – – – – – – – – – – – – – – – – soil
12mg/me
Conversely, if the meq of Mg is known, the mg/100g can be calculated.
Solution: using the above value of 6.67 meq Mg/100g soil. So
6.67 meqMg/100g of soil* 13 mg/me= 80mg Mg/100g of soil.
CONVERSION TO PARTS PER MILLION (ppm)
Example 3: Convert 80mg of Mg/100g of soil to ppm
Solution: convert both the 80mg and 100g to the same unit by multiplying both by 10/10.
80mg. 80mg. 10. 800mg
—— =. – – – – – – *. – – – =. – – – – – – – = 800ppm
100g. 100,000g. 10. 1000,000mg.
TERMINOLOGIES
IONS : Elements having an electrical charge
CATIONS : Positively-charged ions
ANIONS : negatively-charged ions
MILLI EQUIVALENT :is the number of ions which total a specific quantity of electrical charges.
PERCENT BASE SATURATION : This is the proportion of acids and bases on the CEC and can be calculated as follows:
Total meq of bases on exchange sites
% base =(i.e., meq Ca++ + meq Mg++ + meq K+)
saturation ——————————- x 100
Cation exchange capacity
EXCHANGABLE CATION: Exchangeable cations are those that can be exchanged by a cation of an added salt solution.( That is, any added cation will exchange the soil cation)
Exchangeable cations are of two types
1. Acidic cations such as H, Al and NH4. These 3 cations will release H+ ion in solution. That is acidic
2. Alkali or base cations. Such as K, Ca, Mg and Na. These 4 cations will release OH- in solution. That is alkali.

